Scientific Name
Chilabothrus subflavus

The species was redescribed and renamed by Leonhard Hess Stejneger (1851-1943), a Norwegian-born American zoologist working for the Smithsonian Institution. Stejneger held the positions of Assistant Curator for Birds, Curator for Reptiles, Curator for Reptiles & Amphibians and head Curator for biology, a position he kept until his death. He was exempted from retirement by virtue of a presidential decree. He did not provide closer detail to the type locality than Jamaica.

Holotype
USNM: No. 14507, Jamaica, ZSP, 25 Nov 1885.
Type Locality
Jamaica (Jamaica was first called Xaymaca, “land of springs”).
Subspecies
None.
Synonyms
- Serpens major subflavus [336] [pl 274]
- Cenchris } The yellow snake Browne, 1754: 461
- Chilabothrus inornatus : 563 [564] [565] [566]
- Chilabothrus inornatus [104]
- Chilabothrus inornatus [315] [316] [317] [318] [319] [320] [321] [322] [323] [324] [pl iv]
- Chilabothrus inornatus : 3757 [3758]
- Chilabothrus inornatus : 312
- Epicrates subflavus [470]
- Epicrates subflavus : 36
- Epicrates subflavus
- Epicrates subflavus : [325]
- Epicrates subflavus
- Epicrates subflavus : 132
- Epicrates subflavus : 151
- Epicrates inornatus subflavus : 74
- Epicrates subflavus Lynn & Grant, 1940: 117 [118]
- Epicrates subflavus Cochran, 1961: 178
- Epicrates subflavus
- Epicrates subflavus : 260
- Epicrates subflavus
- Epicrates subflavus
- Epicrates subflavus
- Epicrates subflavus [ 2 ]
- Epicrates subflavus
- Epicrates subflavus : 21
- Epicrates subflavus Crother & Crombie, 1999: 67
- Epicrates subflavus
- Epicrates subflavus
- Epicrates subflavus Tipton, 2005: 47
- Chilabothrus subflavus
- Epicrates subflavus
- Epicrates subflavus : 127 [128]
- Chilabothrus subflavus : 15 [16]
- Chilabothrus subflavus : 38
1) Serpens major = Large snake, subflavus = either meaning: yellow, blonde, or "under yellow" . 2) Chilabothrus is from the Greek meaning cheilos "lip", á "without", and bothros "pits". 3) Epicrates is from the Greek Επικρατης meaning "powerful".
Common Name
Jamaican Boa, Yellow Boa, Nanka.

Anecdotal note: Gosse strongly disagreed with the name given to the Jamaican Boa. So much so, he wrote,”The trivial name inornatus, which MM Dumril and Bibron have selected to designate the species, must be considered as comparative, for this boa, when seen alive, in its black and yellow livery, I think, is far from unadorned, the contrast of colours being fine, and the purple iridescent glow that is reflected in the playing light from the “dark parts of its polished armor is very rich and brilliant”. From “A Naturalist’s Soujourn in Jamaica“, 1851.
Taxonomic history
Sloane was the first to describe the yellow snake of Jamaica as Serpens major subflavus. Note that his description occurred before the system of binominal nomenclature was invented by Linneaus and therefore his Latin name for the snake does not follow the system in use today. He did, however, follow scientific standards at the time of his writing and, consequently, he must be included in the synonymy list.

He named two other snakes as Serpens major. Serpens major nigricans – which he described as being “of black color and otherwise everything the same as the yellow snake” and Serpens major cinereus which he described as “of light grey color” . While based on his descriptions Serpens major subflavus is clearly the Jamaican Boa, it remains a bit unclear which species he was referring to by the other two names. Only two other genera occur on Jamaica Hypsirhynchus and Tropidophis, the Jamaican members of both are much smaller than Chilabothrus subflavus.
Crothers (1999) writes of Serpens major nigricans: “Serpens major nigricans” (p. 337) = Alsophis ater Gosse, 1851. “The black snake is only smaller, else in everything the same, al-
though not venemous.” There are two black snakes in Jamaica, one large, Alsophis ater (Gosse) and one small, Arrhyton funereum (Cope). Sloane’s adjective “major” decides the issue. The species ater is probably extinct.”
Ahrenfeldt assumed that the two other snakes named by Sloane are both Hypsirhynchus ater . Considering that Sloane used the term major (= large) and described the species as virtually identical to C. subflavus casts doubt over this interpretation. We therefore disagree with Ahrenfeldt’s opinion and conclude that Sloane indeed described different color forms of Chilabothrus subflavus.

Description and taxonomic notes
The boa was considered to be the same species as the Puerto Rican Boa Chilabothrus inornatus , until Stejneger redescribed it . He used subflavus as the species name, the same term that Sloane had coined to describe the species before him. He made no reference to Sloane’s work, however we strongly assume he must have been aware of it, given the species’ name.
Epicrates subflavus presented a difficulty for Stejneger (1901). There is a difference in color (so vividly described by Gosse), unmentioned by Stejneger except in the usurped epithet, subflavus. Stejneger could find only one constant scale difference between inornatus and subflavus, the contact in subflavus of the prefrontals with the preocular rather than the separation by small scales .
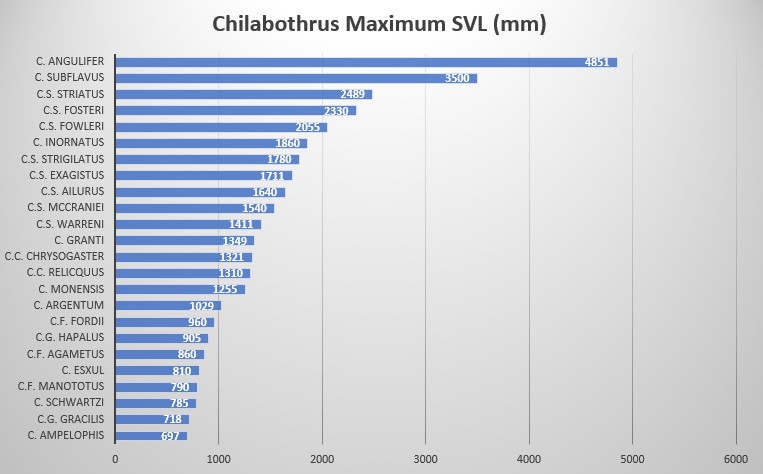
Sloane stated that the snake attains length of seven to eight feet. According to Gosse and Hill, the boa commonly attains a length of eight or ten feet . Note that we are unaware what a foot meant exactly by these authors since the definition of a foot varied and standardization first took place in 1959. By today’s standards Sloane referred to sizes between 213 – 244 cm and Gosse and Hill sizes between 244 – 305 cm. Contemporary accounts state that the maximum SVL is about 200 cm .
The coloration and patterning is very beautiful as was noted already by Gosse and Hill: “The ground colour is yellow, varying from bright golden to a clay-colour, marked with black in irregular spots and confluent bands. These are very few and remote at the fore-parts, but increase posteriorly, the yellow at length disappearing except as scattered spots on an uniform black ground” and “I think, far from unadorned, the contrast of colours being fine, and the purple iridescent glow that is reflected in the playing light from the
dark parts of its polished armour is very rich and brilliant.” . A dark post orbital stripe is present in most but not all animals.

* Source
The relationships of the different Chilabothrus species have recently been re-evaluated. According to the new phylogeny, Chilabothrus subflavus is related closest to C. gracilis and C. fordii. The species has been evolutionary isolated for the last 11.1 million years .
During a study at Marta Tick Cave, Trelawny Parish, Jamaica, fossil vertebrate remains from the late Holocene have been found. While fossils from several reptile species including Tropidophis could be identified, no fossil records of Chilabothrus subflavus could be found at the study site .
Anecdotal note
“Sloane did, in fact, attempt to bring some of these “uncommon Creatures” home to England. He intended to bring the Yellow Snake (“seven feet long”), the Guana, and also a crocodile back from Jamaica alive. There was a tragic conclusion to his effort. While all the animals survived for a while on the ship peaceably and successfully, the snake was shot by servants when it invaded their quarters, the Guana was frightened by a seaman and fell into the sea, and the crocodile died of unknown causes (de Beer, 1953, pp. 47-48). Sloane did not preserve any of these animals nor any of the small ones. The only record of all of these animals are the figures and the text appended. If at least the snake had arrived alive in England, if it had been preserved or even skinned, and if it had been part of collections that Sloane gave to the British Museum, the Yellow Snake of Jamaica would not have had to wait for Stejneger (1901) to give it its formal Latin name, Epicrates subflavus. Stejneger conspicuously did not mention Sloane’s note on the Yellow Boa, although he usurped the
very epithet that Sloane used.”
Distribution
Jamaica and Goat Island (the boa is thought to be extirpated from Goat Island).
The boa did most likely occur on large parts of the island and has been described as common in many parishes . Today Chilabothrus subflavus populates less than 10% of the island and occurs in 20 isolated pockets within Jamaica. Cockpit Country is the largest contiguous habitat for Jamaican boas, followed by Hellshire Hills, Blue Mountains, Portland Bight, and Yallah Mountains . Gibson considered the boa to be possibly extinct from Goat Island, a small island on the south coast of Jamaica . This is corroborated by a later researcher who could also not confirm the existence of C. subflavus on Goat Island .

[intergeo id=”gM2ETM”][/intergeo]
Habitat
On Jamaica the mean annual temperature is 24.91°C and the mean annual precipitation is 2123.08 mm. Peak temperatures occur during summer months of June to September while the coolest temperatures occur during winter between December through March. The Northern portions of the island tend to be exposed to colder temperatures from occasional surges of cool air from continental North America during fall and winter month. There exists a dry season between December through March and a rainy season between April through November which are divided into early rainfall and late rainfall seasons. There exists a mid-summer minimum around July that separates early and late wet seasons. Most of Jamaica’s rainfall occurs during the wettest months May and October and experiences its driest months in March and June. The rainy season, September through December, provides afternoon thunderstorms in west and central Jamaica, whereas the rest of the day is sunny and humid with basking opportunities for boas. Contrary to this the Blue Mountains Region provides frequent misty rains and a closed cloud cover for prolonged periods without the opportunity to bask.



Jamaica has, despite its small size, significant climatic differences between the Limestone Region (west and central Jamaica) and Blue Mountains Region (east Jamaica). The Blue Mountain Peak in the east rises to 2256 meters and receives over 3300 mm of rain. Temperatures in the coastal lowlands average 27°C with the coldest months running about 23°C (January and February) and the warmest months about 28°C (July and August). These regions are both habitat to the Jamaican Boa but differ from their geological history, soil and climate. . The forest cover on the island consists of submontane, degraded submontane, montane, degraded montane, swamp, mangrove and dry.

The boa was first assumed to be found in the “woody mountains” . A habitat use analysis found that several possible habitats are underused. The researchers investigated the reasons for this and concluded that habitats which C. subflavus selects are associated with tall forest and canopy cover. They attribute the underuse of potentially favorable habitats to strong anthropogenic pressures, which are negatively impacting its presence .
Longevity
Like most other Chilabothrus species, C. subflavus is long lived in captivity. Henderson and Powell report 24 years and 4 month and a wild caught male lived 18 years and 11 month at the Fort Worth Zoo, Texas, U.S.A. . 24 years and four months (still alive as of 1997), Dallas Zoo (Slavens & Slavens 2003).
Behavior
The Jamaican Boa seems to be largely arboreal and is dependent on a habitat with closed canopy cover. Vernon describes finding a boa “in the natural reservoirs formed by the spurs of large trees, which sometimes contained more than a gallon of of cool and wholesome water” .

Miersma, using radiotelemetry, studied activity and home ranges of 12 Jamaican Boas (4 males, 8 females) in the community of Windsor, Trelawny Parish, on the northern side of Cockpit Country. She found that the males moved significantly farther per day than females. Mean daily movement was 18.60m ± 2.7SE; females averaged 10.45m ± 0.9SE and that males had much larger activity ranges (10.53ha and 14.03ha) than the females (2.24ha – 3.66ha). .
Several accounts of cannibalism have been documented for this species. Trutnau describes a case from the Ohio Zoo, citing Goode where a large female killed and consumed a male which was introduced to her cage for breeding purposes . Tom Crutchfield found two females fighting (constricting each other and biting) during breeding season. His animals are kept year around in an outdoor enclosure in the garden of his Florida residence. This is the first time that female-female aggression was reported in this species (T. Cructhfield pers. comm.). However, it is unclear what triggered the aggression and if it was linked to the breeding season or if a food item, e.g. wild mouse had been running through the cage and triggered the reflex. M. Søndergard (pers. comm.) lost a female during a breeding attempt, consumed by a male. J. Murray had a juvenile eat a cage mate overnight (see image below). The boa neither regurgitated the eaten boa nor died, as is usual in cases like this.

Schaefer et al. describe five instances of avian mobbing of Jamaican Boas in northern Cockpit Country. In four cases Jamaican Crows (Corvus jamaicensis) were carrying out the mobbing and in one instance a group of smaller Passerines. The authors describe in addition a probable instance of a boa predating a crow’s nest . Avian mobbing has previously been described in the Puerto Rican Boa where large female boas were mobbed . The observations from Jamaica corroborate the previous finding that large boas are mobbed, as four of five mobbing events involved large female Jamaican boas. The researchers speculate that Jamaican Crows and other birds are less likely to detect smaller, well-hidden boas or that smaller boas are less of a threat to a bird the size of a crow and are simply ignored.
Diet
Sloane noted that C. subflavus “feed on Birds, Rats, etc.” and noted that the boas are voracious rat eaters: “Many of them have been kill’d with Thirteen or Fourteen Rats in their Bellies.” . While this might be an exaggeration, the boa was found to be a voracious rat eater also by Gosse and Hill . Vernon describes a nine foot boa raiding a chicken coop and eating three chickens before it was killed . The Jamaican Boa appears to be an opportunistic forager and has been observed within human houses, foraging for rats . The same authors observed a female boa to forage bats in the Windsor Cave. They noted that only one snake was visible at the cave entry over several days and that despite great efforts, she did not succeed in catching a bat. Davalos and Eriksson found Boas attempting to predate bats in Ratbat Hole, however they did not confirm a successful catch by a boa . Koenig and Schwartz confirmed the capture of a bat Artibeus jamaicensis by a juvenile C. subflavus. Despite the fact that several cave systems are visited by boas of different sizes, they found that only juvenile boas attempt to capture bats .

One instance of a young Cyclura collei, the Jamaican Iguana being consumed by a Jamaican Boa has been reported . Avian prey species include fowl and their eggs. A boa captured in a chicken pen was killed and opened. It had consumed seven chicken eggs. These were unbroken and still intact . Black-billed Parrots (Amazona agilis), Yellow-billed Parrots (Amazona collaria) and their nests are supposedly some of the natural prey items of this boa .

The first report of amphibian prey is a tragic one; a C. subflavus had consumed a Cane Toad (Rhinella marina). This species is native to South and Central America, but not to the Caribbean, where it had been introduced to combat sugar cane pests. The species produces the toxin bufogenin, which is often fatal if ingested by species that have not co-evolved with this toad species. The report documents death due to cane toad poisoning in the Jamaican boa .
A large boa (200 cm, 3.6 kg) was captured on 24 July 1980 in St. Thomas Parish and taken to the Hope Zoo. Four fecal samples collected there contained the remains of a large mongoose (Herpestes auropunctatus), an adult rat, boa teeth, and masticated pieces of beetles, ants, and grass. The insects and grass the authors assume, had been eaten by the mammals before they were consumed by the boa. The authors were especially pleased to see that a mongoose was consumed since this introduced species caused (and still causes) such havoc on the Jamaican herpetofauna and the boas in particular .
Reproduction
The first observation of the reproduction of this boa came from F. R. Griffith, communicating it to Gosse and Hill. Griffith noted that he caught a female Jamaican Boa “6 feet 1 inch with a blunt tail” (= 185 cm). The snake was caught on the 3rd of July, 1849, and did not feed on the lizards and mice that were offered to her. She gave birth to twenty-three young on the 19th of October. The young measured sixteen inches (= 40.64 cm) .

Gosse and Hill furthermore report about the mating season occurring in spring and that “when the Yellow Boa pairs, which is in spring, others of the same species approach, and twist themselves with and over the pair, until an immense knot or entwined mass is formed”. If this observation is indeed about mating and not ritualized male to male combat, then this is reminiscent of the breeding balls seen in Eunectes. Laszlo reports the observation made by Huff that Jamaican Boas mate during early March
Bloxam did observe ritualized combat very rarely in C. subflavus but added that combat might have occurred at night time and was thus not observed by staff . Contrary to this, J. Murray observed very intense male to male combats in the species, which is assumed to even end in severe injury or death if opponents are not separated.
Based on information on seventeen birthing events, we calculated the average litter size to be 18.45 young. Litter sizes ranged from two to thirty-four young. Data from the following sources was used: S. Woodward pers. comm., . Huff reported thirty-seven ova which followed a captive breeding attempt but were not developed further . This indicates that the maximum number of young can be much higher if conditions are favorable.

The Columbus Zoo in Ohio USA was most likely the first Zoo to reproduce the species. The Zoo obtained two adult Jamaican Boas in 1968 and had the first litter in 1972 and a second litter in 1977. The Zoo won the national Bean Award for the most significant breeding in 1972 .
Furthermore Slavens & Slavens list captive reproduction records for several zoos and breeders . Click on abbreviation to get information about the breeder/institution. Note that the datapoints do not necessarily represent single breeding events or litters, since several institutions house multiple breeding animals.
1979 JACF 0.0.19 born during 1979.
1985 JACF 0.0.5 born during 1985.
1985 JERE 0.0.22 born in 1985.
1985 RHUG 0.0.55 hatched during 1985.
1985 TOLO 0.0.8 bred during 1985 from a captive breeding.
1986 JERE 0.0.20 born during 1986, 1 did not survive.
1986 TOLO 0.0.18 born during 1986.
1987 JERE 0.0.12 born during 1987, 6 did not survive.
1988 JERE 0.0.32 born during 1988. One did not survive.
1988 LOSC 0.0.6 born during 1988.
1989 JERE 0.0.1 born during 1989.
1990 TOLO 0.0.6 born during 1990.
1990 ZOOR bred during 1990.
1991 TOLO 0.0.22 born during 1991.
1992 TOLO 0.0.2 born during 1992.
1993 CHEEa 0.0.13 born during 1993.
1993 WIEP 0.0.11 born during 1993, 2 did not survive.
1994 JERE 0.0.60 born during 1994.
1996 JERE 0.0.76 born during 1996.
The following litters were produced in 2021:
- US: 17 live on 9 September. Bred by Rob Stone.
- UK: 12 live 2 stillborn and 7 unfertilized ovum on 3 October, 2021. Bred by Tom Middlebrook and Faye Da Costa.
- US: 15 live, 1 unfertilized ovum on 27 September, 2021. Bred by David Muth and Jared Rager.
- US: 34 live, 3 stillborn and 3 or 4 unfertilized ovum on 26 September, 2021. Bred by Tom Crutchfield.
- Germany: 9 live, 1 stillborn and 6 unfertilized ovum on 14 September, 2021. Bred by Sebastian Hölch.
Captive management & population in captivity
A stable, sizable population is present in the collections of American and European Zoos and private reptile keepers. In the USA several private collectors are members of the Species Survival Plan (SSP) and participate in breeding loans with Zoos to ensure the best possible genetic matches between boas are realized.

Through gifting and breeding loans in the Invisible Ark, C. subflavus is present in many collections around the US and in Europe. With the offspring produced by the likes of T. Crutchfield, J. Wagner, one of the authors and many more collectors, responsible breeding of the boas is taking place. If carefully managed, as in the case of the Puerto Rican Boa, the Jamaican Boa will maintain its genetic diversity well into the future.
It is recommended a genetic diversity study of C. subflavus be conducted in the same manner as was the study for the captive population of C. inornatus. The results can be used to inform and manage the reproduction efforts of the Invisible Ark in conjunction with the SSP.
Conservation status, threats and population size in nature
CITES: Appendix I
Jamaica joined CITES on 23 April, 1997; entry on force on 22 July, 1997.
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
USFWS status: Endangered (EN)
-The Jamaican Boa was listed on 2 June, 1970
FWS Focus: No
The population of the Jamaican Boa dramatically declined to near extinction during the last two centuries. This development is directly linked to the history of of the island. The first humans arrived between 6000 and 3000 years before present on the island, followed by Redware people (named after the pottery remains they left) and later the Taino people. Due to their low numbers these had most likely no negative impact on the ecology of the island. The Europeans arrived in 1494 with Christopher Columbus and after the Spanish rule ended around 1660, the British began to exploit Jamaica. This period changed the face of the island significantly. The British used Jamaica to grow sugar cane, coffee, cotton and indigo. The production peaked in the 18th century and with it, the deforestation and transformation of large parts of the land. It must not be forgotten that this production peak was largely based on a terrible treatment of human beings exploited as slaves.
As early as 1754 the Jamaican Boa was used as a food source because of its rich, delicate meat and its oil was considered a good resolutive by the locals (Browne, 1754). At the same time, many boas were killed for no other reason than people were fearful of them. It was known that they are harmless, non-venomous and even reluctant to bite as well as very useful to control rats and mice, which created measurable losses in harvest yields . This paranoid view of nature continued (and still does today), and might have reached its greatest popularity around 1850 as can be seen from the colorful description Gosse and Hill used to narrate an encounter with a boa that entered a house .
Less than 60 years after Gosse and Hill stated that the Jamaican Boa is common on the island, Barbour found it was close to extinction. The main reason for the decline in this short time were nine individuals of the mongoose (Herpestes auropunctatus), which were brought to Jamaica in 1872 to initiate a population that was expected to kill rats and mice. The result was disastrous: mongooses had indeed a significant impact on the rat population but also devastated multiple native avian and reptilian species. Barbour noted that: “Snakes have suffered perhaps more than lizards.” .
The general opinion in Jamaica is that “the Boa, Epicrates, as well as the large Iguana, are now almost extinct on the main island, though they still occur on some of the near-by outlying islets”. . About 60 years later, Mittermeier found that C. subflavus has suffered less than other Jamaican reptiles from the presence of the mongoose. He noted, however, that the boa is killed on sight, has a limited range and is nowhere abundant .
This led to increased conservation efforts including a Species Survival Plan (SSP). Instrumental in establishing a stable captive population was the Jersey Wildlife Trust which obtained wild caught Jamaican Boas from Thomas Huff of Canada in 1976. The offspring of these boas was distributed among zoos and academic institutions as well as private breeders, leading to a large captive population. Tzika et al. investigated the genetic diversity of the European captive population. This captive population originated from 11 (5 males, 6 females) wild caught animals, which were initially incorporated in a breeding program by the Durell Wildlife Conservation Trust. They tracked down 80 of the offspring and found that these originated from only four of the founder animals .
Genetic knowledge about the US captive population in the private sector is currently unavailable and would certainly contribute to a coordinated breeding effort. Advisable is the formation of a stable, diverse ex situ population that would be a georedundent placement of breeder animals. This demands a facilitated exchange between US and European captive bred animals. This exchange is currently impossible due to restrictive interpretation of laws by authorities, which works against the interest of species’ conservation. It is hopeful common sense prevails and measures are put in place to allow the transatlantic exchange of genetics through unrelated offspring.
The most recent study on the Jamaica boa concluded that the boas occur only in a surface area of 9% of the island (1000.6 km²) out of the total area of 10,829 km². The distribution of this species occurs in isolated pockets ranging in size from 0.6 km² to 668.9 km². The fact that the areas are not interconnected poses an additional threat preventing gene flow between populations . Tzika and co-workers studied the population structure of wild C. subflavus from different locations on the island. They identified an Eastern vs. Western and Central pattern of genetic diversity. They speculate that this genetic diversity is an indicator for further different adaptations to the varied ecological challenges the boas are facing in the two habitats. They emphasize the importance of population management and advise that the Blue Mountain and John Crow Mountain populations are managed separately from the populations of the West-Central Jamaica . Further research about genetic variation of the populations and tests of fitness to adapt to the two different habitat types is necessary. For the moment, it is advisable to treat the two lineages as Evolutionary Significant Units (ESUs) and don’t interbreed them, since they might represent adaptations to their specific habitat.
No estimates exist about the population size in terms of numbers. However, given the current threats, habitat destruction, habitat fragmentation, persecution by humans and introduced species (namely mongoose, pigs, cane toads), we believe the wild population is under severe threat. Currently no studies exist to measure the effect of human caused climate change on the Jamaican Boas’ survival, however, the effect of climate change on the islands of the West Indies itself has been well modeled and the outcome of these studies paints a bleak picture.
It is estimated that for the period 2071–2100, temperatures will increase across the region by 1–4 °C for all months irrespective of the scenario. Rainfall patterns will change as well, with the northern West Indies (i.e. north of 22°N) becoming wetter and the southern West Indies will receive less rainfall . Most likely seasonality will be largely affected .
Despite the difficulties and challenges from local to global level, it is encouraging to see that conservation efforts are being made on Jamaica ranging from public education, habitat restoration to short distance translocation of the boas. It is mandatory to prevent further habitat fragmentation and preserve genetic diversity in situ. In addition, the ex situ efforts to keep a self sustaining, diverse captive population representing both ESUs is a very important task that citizen conservationists as well as zoo professionals must approach in a combined effort, using the SSP when possible.
We predict this boa will be removed from the ESA in the years to come. Through the efforts of the Invisible Ark individuals such as J. Murray, T. Crutchfield, J. Wagner, S. Woodward, et al., this boa is now present in almost every state in the US. Through gifting and breeding loans this boa has made an amazing recovery and, in the process, has managed to maintain its genetic diversity through responsible breeding. The USFWS has seen fit to make exceptions to the Lacey Act with regard to the CITES I/App I Madagascan Boas (Sanzinia & Acrantophis) as well as several App I tortoises. Affording the Jamaican Boa and Puerto Rican Boa the same exceptions would allow the free trade of genetics and blood lines across state lines without the ensuing red tape that makes it impossible to do so. This is a very reasonable and scientifically sound recommendation on the part of the authors.
Anecdotal note
On 16 April, 1984 Crombie et al., explored Cabarita Island, Saint Mary Parish off the north shore of Jamaica. They found two mammals, five reptiles, two amphibians and thirteen birds during their sampling of the island. Of particular note is this passage regarding Rattus cf. rattus: “Rats were extremely abundant on the island, but we were unsuccessful in trapping any. Rat traps, especially those set on tree limbs or or [sic] horizontal trunks, were sprung during the evening but the rodents escaped. Nocturnal surveys revealed dozens of rats, usually in the canopy of the forest but several were seen near the garden plot. Throughout the night, our bodies and gear were subjected to intense investigation by adult and juvenile rats.”
The CIA World Factbook lists the following environmental threats for Jamaica: heavy rates of deforestation; coastal waters polluted by industrial waste, sewage, and oil spills; damage to coral reefs; air pollution in Kingston from vehicle emissions; land erosion .
The map below illustrates the extent of habitat destruction and alteration due to development and agriculture.


On display in these Zoos
The Jamaica Boa is on display in several Zoos. The most accurate listing of species on display in European and Russian Zoos, can be obtained from the Website Zootierliste.
Jaw-dropping Jamaicans