Scientific Name
Chilabothrus inornatus

Described and named by Dr. Johannes Theodor Reinhardt (1816-1882), a Danish zoologist and Curator of Terrestrial Vertebrates at Det Kongelige Naturhistoriske Museum, Copenhagen.
Holotype
Syntypes: Universitetets Zool. Mus. Kobenhavn (UZM) R.5597-98 and R.55101. Stimson presumes the latter is lost .
Type locality
Puerto Rico.
Synonyms
- Boa inornata [254] [255] [256] [257] [fig 21-23]
- Boa inornata Troschel, 1843: 223
- Chilabothrus inornatus Dumeril & Bibron, 1844: 563 [564] [565] [566] *
- Chilabothrus inornatus (Gray, 1849) [104]
- Chilabothrus inornatus Hinrich Lichenstein, 1856: 23
- Chilabothrus inornatus Jan, 1863: 24
- Chilabothrus inornatus Jan, 1864: pl V
- Chilabothrus inornatus Jan, 1865: 87
- Chilabothrus inornatus : 312
- Piesigaster boettgeri Seoane, 1881: 217 [218] [219] [220] [221] [222] [223] [224] [Tab I] ** [erroneous]
- Piesigaster boettgeri La Ilustracion Espanola, 1882: 125 [Front page] (erroneous)
- Piesigaster boettgeri Boettger, 1886: 115 ** [erroneous]
- Chilabothrus inornatus : 279
- Epicrates inornatus Boulenger, 1893: 97
- Epicrates inornatus [63]
- Epicrates inornatus [689] [690] [691] [692]
- Epicrates inornatus : 325
- Epicrates inornatus
- Epicrates inornatus : 130 [131] [132]
- Epicrates inornatus : 141
- Epicrates inornatus : 108
- Epicrates inornatus Grant, 1932: 344
- Epicrates inornatus : 327 [328] [329] [pl 38]
- Epicrates inornatus : 224 [225]
- Epicrates i[nornatus]. inornatus Stull, 1933: 1 [2]
- Epicrates inornatus inornatus : 131
- Epicrates inornatus inornatus
- Epicrates inornatus inornatus : 150
- Epicrates inornatus inornatus : 74
- Epicrates inornatus inornatus
- Epicrates inornatus [96] [97] [98]
- Epicrates inornatus
- Epicrates inornatus
- Epicrates inornatus Rivero, 1978: 101 [102]
- Epicrates inornatus
- Epicrates inornatus
- Epicrates inornatus
- Boella tenella [20] [21] [22] [23] [24] [25] [26] [27] [28] [erroneous]
- Epicrates inornatus [163] [164] [165] [166] [167] [168] [169] [170] [emendation]
- Epicrates inornatus
- Epicrates inornatus Liner, 1994: 22
- Epicrates inornatus : 21
- Epicrates inornatus
- Epicrates inornatus Tipton, 2005: 45
- Epicrates inornatus : 138
- Chilabothrus inornatus
- Epicrates inornatus
- Chilabothrus inornatus : 13
- Chilabothrus inornatus : 38
* Chilabothrus is from the Greek meaning cheilos "lip", a "without", and bothros "pits." * The name inornatus is derived from the Latin for "Unadorned." ** Boulenger (1893) listed P. boettgeri in synonymy. Stejneger (1904) correctly identifies Seoane's specimens as C. inornatus. The specimens were provided to Seoane by his brother, an officer in the Spanish navy. Vanzolini (1977) draws attention to the fact this is Epicrates inornatus, thus making it West Indian.
Common name
Puerto Rican Boa, Culebron.
Description and taxonomic notes
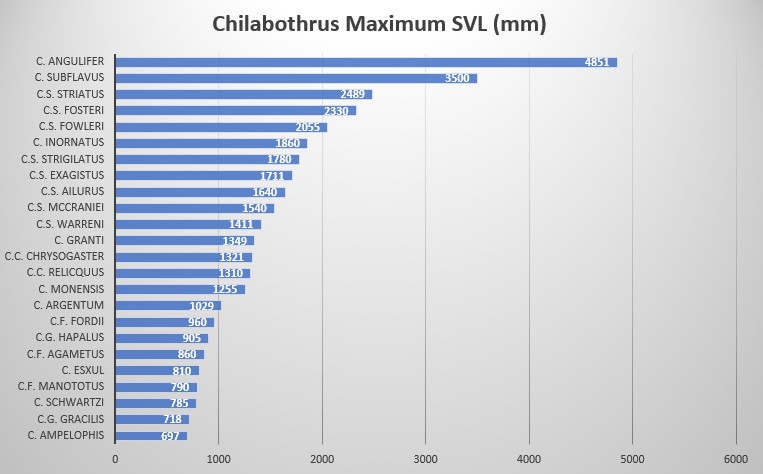
SVL is to 1860 mm in males, 1750 mm in females; ventrals measure 263-273 in males, 258-267 in females; subcaudals are 66-74 in males, 68-75 in females; ventrals + subcaudals range 329-338 in males, 326-339 in females; intersupraoculars 1 in males and females; pre-intersupraoculars 0 in males, 2-3 (2 modally) in females; post-intersupraoculars 2,3 (2 modally) in males and females; dorsal scales at the neck 31-34 ; dorsal scales at midbody 38-42; dorsal scales anterior to vent 21-25; supralabials 11-12 (11 modally); infralabials 11-14 (12 modally); supralabials entering the eye 5-7 (6 modally); circumorbitals 7-10 (8 modally); loreals 1-2. Neonate SVL is 332 mm-367 mm .

C. inornatus is a larger boa with light brown/dark brown coloration with a well defined pattern. Some specimens exhibit a grayish coloration. Reddish and yellowish base color specimens have also been reported from small areas (Diaz pers. comm.). The overall coloration is marked with dark brown angulate blotches that may have black outlines. Some specimens are more vividly marked than others. Some specimens may even exhibit little or no markings and appear as dark brown overall, especially as they age.
Wiley observed a large Puerto Rican Boa of 241 cm total length (205 cm SVL) , which is most likely the upper size limit for the species. Most C. inornatus reach lengths of up to 186 cm SVL. Females tend to outgrow males of the same age. Puerto Rican Boas are most closely related to the Mona Boa and the Virgin Island Boa; their lineage split about 10.5 Mya. The next closest relative to the clade is C. angulifer; the lineages split about 15.3 Mya .

* Source

Distribution
Puerto Rico.
With a total area of 8900 km², Puerto Rico is the smallest of the Greater Antilles. It is divided in three physiographic regions or areas of relief: the mountainous interior, the karst region, and the coastal plains and valleys. The island comprises six ecological life zones: subtropical dry forest, subtropical moist forest, subtropical wet forest, subtropical rain forest, lower montane wet forest and lower montane rain forest . The boas are found from sea level to 1050 meters.
The Puerto Rico Area consists of approximately 200 islands and cays. All these were last connected during the Pleistocene with the lowering of sea levels, leading to a single elongate island that extended from Puerto Rico to Anegada having an area of around 21,000 km2 . Interestingly, Chilabothrus inornatus does not occur on the previously connected cays but only on the main island of Puerto Rico .

C. inornatus is widely distributed throughout Puerto Rico; the US. Fish and Wildlife Service lists its presence for 62 of the 83 municipalities of Puerto Rico . It is especially abundant in the northern karst region . This species has an elevation range of sea level to approximately 1050 meters (El Yunque) above sea level .
A study listed the Puerto Rican Boa as an introduced species in Florida, based on a single animal that was dispersed with a shipment of garbage from Puerto Rico. This might have been one of very few incidents in which the Puerto Rican Boa was introduced to Florida. Currently there is no evidence of an established population outside its natural habitat .
[intergeo id=”QO0ETM”][/intergeo]
Habitat
Puerto Rico’s mean annual temperature is 24.84°C and mean annual precipitation is 2115.39 mm. Temperatures range from 70 to 90 °F (21 to 32 °C) in the lower elevations, while higher elevations in the central part of the island experience temperatures between 61 and 80 °F (16 and 27 °C) year-round. The forest types that comprise the Puerto Rican landscape are moist coastal forest, moist limestone forest, dry coastal forest, dry limestone forest, lower cordillera forest, upper cordillera forest, lower luquillo forest and upper loquillo forest . In the 16th century “the Body of the Tree Zoyla is so thick, that fifteen Men holding Hands together, cannot encompass the same” (Ogilby, 1671).

The boa is found in a variety of habitats. It occurs in rain forest, karst landscape, caves (western and central Puerto Rico), dense tropical deciduous forest and altered environments such as plantations and urban areas. Grant states the boas are arboreal and return to the ground on rocky terrain. He reported 2 boas at 700 meters from the headwaters of the Rio Mameyes and on 10 March, 1931 collected a “nice specimen at Piedra Blanca Cliff, at about 1500 feet elevation on the Luquillo Range, near the northeast corner of the island” . Rivera indicates they inhabit trees and caves . Areas disturbed by human habitation, tree plantations, buildings and forest edges have all produced sightings of C. inornatus.

A radio telemetry study following a small number of boas (n=18) throughout a year found them most often located in live broadleaf trees (52.8%) followed by ground or below ground sites (34.9%). Other sites less frequently occupied included vine enclosed broadleaf trees and shrubs (6.5%); vine tangles (1.7%); sierra palm, Prestoea acuminata (1.0%); tree ferns, Cyathea spp. (0.8%); bamboo, Bambusa vulgaris (0.8%); dead trees (0.7%); stream (0.4%); building (0.3%); and miscellaneous cultivated plants (0.1%) .
Several Facebook groups, dedicated to the Caribbean Boas or the Puerto Rico native fauna in general, regularly document the abundance of C. inornatus and the frequent encounters with humans. By all appearances the boa has managed to live in urban and disturbed habitat, with people finding newborn C. inornatus where they live. This is a good indicator of the tenacity of this large boa and its ability to adapt to altered habitats.

Longevity
C. inornatus is long lived, reaching at least 28 years of age. J. Wagner is in possession of a Zoo bred female born in 1992, making her 28 years old (Wagner, pers. comm). Murray has 2.2 boas from two Zoos; the boas were born in 1996; making them 24 years old. Despite this advanced age, all the boas are still reproductively active.
Reproduction
Slavens reports the following reproduction data:
1979 JACF 0.0.16 born during 1979.
1980 OKLO bred during 1980.
1985 RHUG 0.0.26 born during 1985.
1985 SACC 0.0.11 born during 1985 from a captive breeding.
1986 JERE 0.0.33 born during 1986, 13 did not survive.
1986 KNOT 6.6.0 born during 1986.
1987 JERE 0.0.19 born during 1987.
1987 SACC 0.0.9 born Aug 21st.
1988 JERE 0.0.14 born during 1988. Two did not survive.
1989 JERE 0.0.10 born during 1989. 1 did not survive.
1989 SACC 0.0.6 born during 1989.
1990 TOLO 0.0.11 born during 1990.
1990 ZOOR bred during 1990.
1991 JERE 0.0.3 born during 1991.
1992 CENC 0.0.6 born 29 Dec 92, 5 did not survive.
1996 BALM 0.0.18 born 26 Sep 1996.
Thomas Huff (Canada) found that Puerto Rican Boas mate in March and April . His animals produced 12-14 young on average, much fewer than reported by other breeders or the wild animals observed. Reagan states mating occurs during the wet season, April and May . Grant collected two specimens May 17, 1931, which mated the same day while being transported in a box. After the snakes were sacrificed, July 20, 1931, the female was found to contain 32 embryos . Other matings in the wild were observed on 6th and 15th of May .


Vergner reported two breedings of C. inornatus in Czechoslovakia. The animals mated from May until June and births (both times 12 young) took place in December . Bloxam and Tonge (Great Britain) found a large variability in the initiation of the mating period, from February through April . This is most likely owed to different weather patterns in the different years.
Multiple males, though not necessary, are helpful in captive breeding programs to enhance fecundity and litter size. Neither J. Murray nor other recent breeders of the species observed harmful male to male combat during courtship and mating. The males will intertwine their heads and necks and push each other before turning their attention to the female. While one male mates with the female the other males will court her by spurring her back and throwing coils on top of her.

In contrast to this, Huff stated that when the males are introduced they react by simultaneously releasing a strong smelling musk and constricting one another.. It is thus unclear whether Chilabothrus inornatus has a stereotypical mating behavior with male-male combat, or if the individual behavioral patterns of some animals lead to this observation. In contrast to C. subflavus or C. strigilatus the behavior observed by Huff did not result in (lethal) injuries. Bloxam and Tonge (who received animals from Huff, including 2.2 of the original breeding group) observed male-male combat like Huff did before them .

Wiley observed several gravid females in his study, four of which were smaller than the minimum breeding size (160 cm SVL) reported by Huff. The smallest female containing embryos in this study measured 108 cm SVL and had a mass of 685 g. The gravid boas observed by Wiley contained 13-30 embryos, in addition he reports the largest litter (observed by F. Valentín Rodríguez), which consisted of 37 young. This is the maximum observed litter size for C. inornatus . Litter size ranges from 12-37 young, with the babies measuring 275-440 mm long. Gestation is approximately 180 days long. In captivity females will consume unfertilized ovum and stillborn young.
Most records of active boas in the Luquillo Mountains have been made between March and June, and none have been made between October and December. Peak activity seems to be during periods of rain immediately following prolonged dry spells. This period of maximum activity is at the time when seasonal temperatures, day length, and rainfall levels are increasing . This aligns with what private breeders are familiar with when cycling their boas for breeding season.

Behavior
Relatively few data exists on behavior in the wild. A telemetry study on 18 animals revealed activity patterns and found an increase in male boa movements from April through June. The researchers believe that males actively search for females at this time. The female movements peaked in July following the male peak, potentially due to increased foraging to sustain embryo growth as well as a shift to environments appropriate for gestation and parturition. The researchers note that the April to July peak in boa movements also approximately corresponded to the period of reproductive activity in some boa prey at the research site .

In captivity the young will strike and, with open mouth, swing their head and neck left and right until they come in contact with the offered food item. They eventually grow out of this innate behavior once they become accustomed to their enclosure and feed regimen. Unless C. inornatus is handled from a young age, and quite often, they tend to remain irritable and unpredictable. The boa will often tolerate the keeper’s presence for a very short time and then suddenly lunge in the direction of the keeper. The boas have an exceptional reach when striking.
A case of cannibalism was reported, where a ca. 100-150 cm TL animal constricting and preying upon a conspecific juvenile (ca. 50 cm TL) in a karst valley in Sabana Seca . Interesting are two obervations made by Rodriguez-Duran, first is the piracy of food (a bat). The author reports that the snakes didn’t bite one another, and the fight consisted of pulling different ends of the bat and tangling their bodies. The second observation relates to the timing of boas. The snakes began arriving at the cave several minutes before the bat exodus to over 30 min after the onset of bat activity . It is remarkable that the snakes obviously learned time patterns of the bats and positioned themselves prior to the event of mass exodus.
While adult boas have been reported as having few natural predators , it is interesting that at least seven species of birds (two winter residents and five permanent residents) were mobbing boas. The advantages of mobbing for the birds are: driving away predators and helping birds improve their predator detection and recognition skills. While avian mobbing of large sized Puerto Rican Boas appears relatively ineffective at driving them away, it may help inexperienced birds to associate danger with the mobbing vocalizations and to learn to recognize predators .
Diet
Adults are opportunist feeders and take rats, mice, birds (including chickens) . Predation of bats has been observed in this species , similar to many other neotropical Boas . A study conducted in the Cueva de los Culebrones confirmed the previously reported bat predation by the boas but found as well the first report of carrion feeding. On four instances, boas were observed feeding on dead Erophylla sezekorni lying on the ground. One of these observations involved a stiff, dried bat. . Wiley analyzed the stomach contents of 29 Puerto Rico Boas and found Black Rats (Rattus rattus) were the most common prey item (61.8%). The author also made the observation of a boa preying on one egg and one hatchling at a cattle egret (Bubulcus ibis) nest at a cay in Bahía Montalva . A recent observation of C. inornatus constricting an Iguana took place at Monte Pirata, Puerto Rico.


Here is a video link of a cave system with C. inornatus taking a bat.
In captivity the adults take a variety of prey items, from chicks to appropriately sized rats and mice. The young take anoles as a first food, with the rare baby starting out on hot pink or fuzzy mice as a first meal. The young take anolis lizards and arboreal tree frogs in captivity . Regardless, the babies switch to rodents very quickly compared to the majority of other species in the genus.
Captive management and population in captivity
C. inornatus has become quite popular in captive collections in both the US and Europe. They are readily available every year as a result of captive breeding programs by people with special interest in the West Indian Boas. C. inornatus do very well in captivity and readily breed, producing litters every other year. The Puerto Rican Boa is well represented in collections in the US and the EU.
A genetic diversity study of US C. inornatus in private collections (and several zoos) was conducted by the Department of Biology, UNC in 2019-2020. The study concluded the boas in American collections exhibited a high genetic diversity relative to wild populations. There are 13 females, as well as a “sizable” number of males, that constitute the majority of the diversity and founder stock of the boas in collections and Zoos. These data demonstrate that ex situ US lineages of C. inornatus are more diverse than expected and that the population retains a higher‐than‐expected number of haplotypes and relatively low signature of loss of heterozygosity . This study demonstrates, and gives credence to, the effectiveness of the Invisible Ark.
Conservation status, threats and population size in nature
U.S.A. joined CITES on 14 January, 1974; entry into force on 1 July, 1975.
IUCN RED List: Least Concern (LC) (click here)
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
USFWS status: 1 (click here) 2 (Click here)
Federal Register: (click here)
C. inornatus is protected by the Endangered Species Act of 1973. A recovery plan was put in place in 1983. The species’ status was changed to Vulnerable (VU) in 2004 by the Department of Natural and Environmental Resources, yet it is still considered Endangered (EN) by the U.S. Fish and Wildlife Service. The Puerto Rican Boa has never had a Species Survival Plan (SSP), unlike the Jamaican Boa.
The Island of Puerto Rico is the smallest island of the four greater Antilles, having a land surface area of approximately 8,900 km2. Three main geomorphologic areas are present; the central interior mountain region, the discontinuous coastal plains, and the karst region of the northwest and north-central land areas. At the beginning of the 20th century Puerto Rico was almost completely deforested (80%) owing to the political and economic influence of the United States. Puerto Rico was one of the principal suppliers of sugar cane to the US, resulting in the conversion of large parts of the island’s forests to sugar cane production and to a lesser extent tobacco. By the middle of the century, as little as 34 km2 virgin forest remained. When the sugar cane industry collapsed about 70 years ago much of the land devoted to the industry was abandoned. Since then there has been an impressive increase in the amount of forest cover .
Puerto Rico’s history of protecting areas for conservation purposes goes back to 1876, when the Spanish Crown protected areas in El Yunque and Utuado. Laws of the Spanish Crown protected forests, mangroves and water resources. Currently, an important face for the conservation of land and biodiversity on the island is the Puerto Rico Conservation Trust, a private corporation. Since 1970 it has managed to acquire and protect twenty natural areas for conservation for a total of 21,364 acres (86.5 km² ) .
Conversely, US policies also contributed to the protection and conservation of land and biodiversity mostly in the Luquillo mountains in eastern Puerto Rico . The Puerto Rican Boa survived these changes but was considered at the brink of extinction around 1970, when the species was listed on the U.S. Endangered Species Act of 1973 and placed on CITES Appendix I. This species has undoubtedly been threatened in the past and declined in numbers. Reasons for declines aside from the mentioned land cover change were also the killing by humans for snake oil, which was in great demand at the time .
Regarding the use of snake oil, Grant (1933) remarks, “Snake oil is in great demand for “medicinal” purposes, but the fat must be extracted from a living snake. Much hog fat is sold by the reputed snake oil sellers. The hind part of the body of my six foot specimen held about two cups of very soft, fine fat” .
Today Puerto Rican Boas might be still killed by humans (even though we are unaware if snake oil is still used) and it is predated on by introduced mongooses. The human alterations on the Puerto Rico landscape are severe and due to an increasing population, this trend will most likely continue. A stronghold for Puerto Rican Boas are inaccessible karst areas, which this species prefers. However the karst areas are considered a vital resource for Puerto Rico and its economical Importance is being recognized (water, minerals, agriculture and forestry) . Thus potential future conflicts between man and snake are lurking under the surface. Nonetheless, this species is described as common in undisturbed karst areas of northwestern Puerto Rico .
Much of the boa’s apparent rarity undoubtedly relates to observers’ difficulties in visually detecting the species in forests and this boa is not as rare as previously thought . Although the species is probably less abundant than it was in pre-Columbian times, recent accounts indicate that it is still widespread on Puerto Rico, and suggest that it may be common in some locations .
The IUCN justified its 2010 rating of LC with the following:
Chilabothrus inornatus is assessed as Least Concern due to its large distribution, lack of widespread threats, and ability to inhabit altered environments. Population numbers have declined in the past but this boa is still relatively common in many areas, and occurs in several protected areas.
This abundance in natural and altered habitats was recognized; so much so, a CITES study submitted in 2016 found the boas had made a sufficient recovery and recommended delisting the Puerto Rican Boa from Appendix I and relisting it as Appendix II. The study documents:
i) given the absence of international trade, (ii) given that it is the only endemic boa therefore trade in other similar species is non-existent, and (iii) given its common and widespread occurrence on Puerto Rico, we conclude that the species does not meet the criteria indicated in Resolution Conf. 9.24 (Rev. CoP16) Criteria for amendment of Appendices I and II and should be moved from CITES Appendix I to II.
The USFWS, via the Federal Register seen here, requested comments from interested parties as it considered removing the Puerto Rican Boa from the Federal List of Endangered and Threatened Wildlife on 13 July, 2022. As of May, 2023 there has been no decision published by the USFWS.
During the recent CITES conference held in Panama City, Panama from 14-25 November, 2022 it was agreed by the Conference of Parties (CoP) the Puerto Rican Boa, Chilabothrus inornatus be downgraded from CITES Appendix I to Appendix II, effective 23 February, 2023. This Notification to the Parties can be found here. We found the USFWS Bulletin somewhat vague regarding the bullet point for Chilabothrus inornatus and sent an email to the agency requesting clarification. The agency’s response (pers comm) read, “The CITES reclassification does not affect the ESA listing in any way. U.S. trade with the species would still need to meet the ESA Import/ Export Requirements. The CITES listing only affects international trade so there are no changes to its interstate movement or sale at this time.”
Despite the fact that Puerto Rico has a long history of protecting areas for conservation purposes, dating back to 1876, when the Spanish Crown protected areas in El Yunque and Utuado, the current situation leaves room for improvement. A study by Castro et al. analyzed a total of 95 protected areas that represent 8.2% of the land surface area. The majority of the protected sites were rather small (often less than 10 km2). The protected areas are effective in conserving species because they are located within the most species-rich regions on the island and because they include sites classified as critical wildlife areas or important bird and biodiversity areas. Additionally, they encompass diverse landscapes, are dominated by core forest and include predicted habitats for 31 threatened vertebrate species .
The percentage of land surface protected lags behind the UN Millennium Development Goals for 2020, which seek to protect 17% of terrestrial areas and 10 percent of nationally administered marine areas (http://www.cbd.int/sp/targets/). The problem of isolation of the protected areas must not be underestimated, since it could lead to a fragmentation of populations which might in the long term lead to inbreeding due to the lack of exchange of genetic material. Puerto Rican Boas are capable of tolerating human activity in their proximity and might even profit from rodents cohabiting human populated areas. However, this is largely dependent on the type of landscape alteration as well as the pressure placed upon the boas when encountering humans.
The latter point can be addressed by wildlife education; the Puerto Rico Conservation Trust, a private corporation, offers excellent interpretative nature programs at Las Cabezas de San Juan Natural Reserve in Fajardo, Hacienda Buena Vista in Ponce and in La Esperanza en Manatí . The former point is largely dependent on cultural aspects like natural gardens but also on agricultural practices. In addition, habitat restoration projects are taking place in Puerto Rico and show very encouraging results . Boas are colonizing the reforested areas, following the increase in prey species. The overall herpetofauna density increased from 17 individuals per ha in the pre-reforested site to 1339 individuals per ha in the reforested and 1361 individuals per ha in the reference site .
The CIA World Factbook lists the following environmental threats for Puerto Rico: soil erosion; occasional droughts cause water shortages; industrial pollution .
We predict this boa will be removed from the ESA in the years to come. Through the efforts of the Invisible Ark individuals such as J. Murray, T. Crutchfield, J. Wagner, S. Woodward, R. Stone, etc. this boa is in almost every state in the country. Through gifting and breeding loans this boa has made an amazing recovery and, in the process, has managed to maintain its genetic diversity through responsible breeding. The USFWS has seen fit to make exceptions to the Lacey Act with regard to the CITES I/App I Madagascar Boas (Sanzinia & Acrantophis ) as well as several App I tortoises. Affording the Puerto Rican Boa and Jamaican Boa the same exceptions would allow the free trade of genetics and blood lines across state lines without the ensuing red tape that makes it impossible to do so. This is a very reasonable and scientifically sound recommendation on the part of the authors.
The map below illustrates the extent of habitat destruction and alteration due to development and agriculture.


On display in these Zoos
Several zoos keep Chilabothrus inornatus. For holdings in European and Russian Zoos see here. Unfortunately most non-reptile enthusiast visitors to zoos fail to recognize the beauty of this species. So much that in fact C. inornatus was the second least attractive boid species (surpassed only by C. gracilis), which negatively affects the holdings of the species in public institutions .
Intriguing Inornatus