Scientific Name
Chilabothrus fordii

Described and named by Albert Charles Lewis Gotthilf Gunther (1830-1914) in honor of George Henry Ford (1809-1876). Gunther is ranked the second most prolific reptile taxonomist. South-African born Ford was an artist at the British Museum known for his excellent herpetology drawings.
Holotype
BMNH 1946.1.1.55, Port-au-Prince, Department de l’Ouest, Haiti. Cap-Naitien and Gonaives, Haiti. 3 syntypes lost except for HZM 52.
Type Locality
Type locality: “Western Africa”; restricted by Sheplan and Schwartz, 1974, to the vicinity of Port-au-Prince, Département de l’Ouest, Haiti, but corrected to “República Dominicana” by . Unfortunately 3 of the syntypes of C. maculatus have been destroyed .
Synonyms
-
- Pelophilus fordii : 142 [pl. 23]
- Pelophilus fordii Gunther, 1861: 189
- Chilabothrus fordii : 87 [88]
- Chilabothrus maculatus : 33 [34] [35] [51] [tab III]
- Epicrates fordii : 98
- Epicrates fordii [64]
- Epicrates fordii
- Epicrates fordii
- Epicrates fordii : 140
- Epicrates fordii : 108
- Epicrates fordii fordii
- Epicrates inornatus fordii
- Epicrates fordii fordii : 150
- Epicrates inornatus fordii : 74
- Epicrates inornatus fordii [319] [320] [321]
- Epicrates inornatus fordii
- Epicrates fordii fordii
- Epicrates fordi fordi [25] [26] [27]
- Epicrates fordi : 110
- Epicrates fordi fordi : 45
- Epicrates fordi : 93
- Epicrates fordi fordi
- Epicrates fordii
- Epicrates fordii
- Epicrates fordi fordi : 19
- Epicrates fordii : 14 [15] [16] [17]
- Epicrates fordii : 113 [114] [129] [157] [158]
- Epicrates fordii
- Epicrates fordii
- Epicrates fordi : 45
- Epicrates fordii fordii : 132 [134] [135]
- Chilabothrus fordii
- Epicrates fordii : 132
- Chilabothrus fordii : 11
- Chilabothrus fordii : 38
Subspecies
- Chilabothrus fordii fordii
- Chilabothrus fordii agametus
- Chilabothrus fordii manototus
Common Name
Ford’s Boa, Hispaniolan Desert Boa.
Description and taxonomic notes
C. fordii is sexually dimorphic. Dorsal scale rows at neck are 28-35, dorsal scale rows at midbody are 31-39, ventrals are 231-261 in males, 236-259 in females, subcaudals in males are 69-85, 70-89 in females, ventrals+subcaudals are 308-343 in males, 312-341 in females, preintersupraoculars are 3, intersupraoculars are 1, post-intersupraoculars are 3. Light gray to gray-tan dorsal ground color. 58-88 dark dorsal body blotches. Neonate SVL is 250 mm . Neonate C.fordii (and C. monensis) are the only members of the genus born with a gray or grayish-brown ground color .


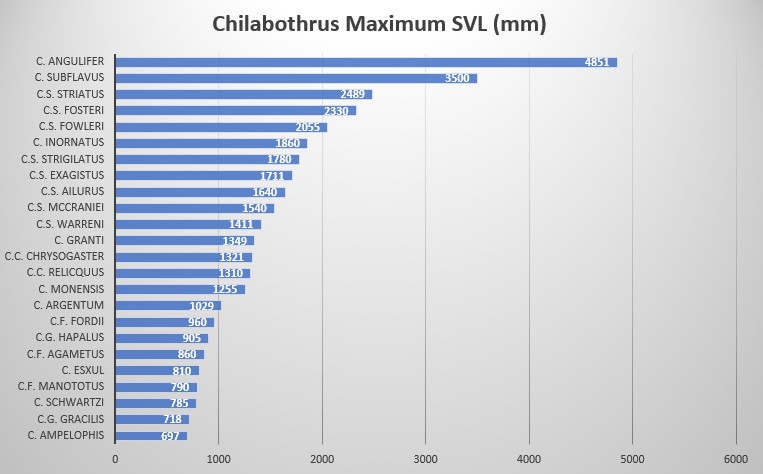
Chilabothrus fordii is one of the smallest species within the genus. While Tolson stated that the maximum size is 860 mm SVL , Bulian reported a specimen of about 130 cm that he had seen at a Miami reptile dealer. His own largest male reached 960 mm total length . The animals which reproduced several times at the Dallas Zoo measured 820 mm (female) and 760 mm (male) in total length .


* Source
Should a population of C. fordii (either nominate ssp. or C. f. manototus) have existed on Ile a Cabrit, it is possible that the population is extinct due to massive habitat change on the islet for development purposes.
The Ford’s Boa puzzle is not yet fully solved:
Ford’s Boa appears to occur on various places in Hispaniola, however, it is likely the most geographically restricted of the three Chilabothrus species occurring on Hispaniola . Schwartz was the first author to tentatively point out that some populations currently considered to be C. f. fordii might be indeed new subspecies . He wrote:
There are also apparently isolated populations at Cap-Haitien on the northern Haitian coast and in the Valle de Cibao in northwestern Republica Dominica; these 2 populations are questionably assigned to E.f. fordii (Sheplan and Schwartz). A third isolate has recently been discovered on Isla Catalina by R.I. Crombie (pers. comm.). That island is far (135 km) to the east of the previously easternmost locality record (Limonal) for the species.]
Schwartz and Henderson pushed the idea of novel subspecies further as they wrote :
"Epicrates fordi is known also from the northern Dominican Valle de Cibao (between Monte Cristi and Villa Vasquez); the population remains unassigned sub-specifically."
Tolson and Henderson put question marks on their distribution map of C. fordii indicating that some populations in the north of Hispaniola might need subspecific assignment . While the current situation makes it hard to justify the status of the subspecies agametus and manototus, due to the very close pholidic, chromatographic and overall morphological resemblance, we consider it possible that several subspecies might indeed exist within C. fordii.
Previously molecular analysis has revealed great diversity within species which were previously considered a single evolutionary unit e.g. Morelia viridis , Corallus caninus and others. Thus, we consider it necessary to analyze morphologically, as well as genetically, C. fordii from different localities across the range to clarify the subspecific question and identify where intergradations, if any, occur.
Problematic in this respect is the scarcity of museum specimens and often the impossibility to use these for genetic testing. Another challenge is the political instability on Haiti which makes it difficult, if not impossible, to access all necessary locations. This should be accomplished as soon as time, resources and safety allow for the collection of data. This is a race against habitat destruction that we cannot afford to lose.

Distribution
Hispaniola: de Cul de Sac-Valle de Neiba plain, Llanos de Azua Gonaives, Île de la Gonâve; Ile a Cabrit in Haiti and the Dominican Republic. Isla Catalina , Isla Saona are the two easternmost localities from which this species has been collected. Found from below sea level to about 305 meters above sea level.
Known from four general regions: the Cul de Sac-Valle de Neiba plain and the eastern Llanos de Azua, northward to Gonaives in Haiti; the Valle de Cibao in northwestern Domincan Republic; Cap-Haitien in north and central Haiti. Mole St. Nicholas on the Presqu’ile du Nord-Ouest. Not found on the south island with the exception of along the northern slope of the Morne l’Hopital in Haiti and the Sierra de Baoruco in the Dominican Republic .
All three subspecies occur on different regions of the island Hispaniola (Haiti, Dominican Republic). Several intergradation zones have been documented.
[intergeo id=”ANzETM”][/intergeo]
Three subspecies of Chilabothrus fordii occur on Hispaniola. The Flags on the map indicate a type locality of each subspecies. Scroll over to see which subspecies occurs at the indicated point.
Habitat
Hispaniola consists of Haiti (the “hilly country”) and the Dominican Republic. There are four main mountain ranges on the Dominican Republic side that parallel each other while running northwest to southeast. Cordillera Septentrional is the most northern range. Cordillera Central has the highest point on the island, Pico Duarte at 3087 meters, and extends into Haiti. The two ranges in the south, Sierra de Bahoruco and Sierra de Neiba, rise in excess of 2000 meters. A smaller range, Cordillera Oriental, rises to 600 meters and is also in the northeast. A majority of the island is over 1000 meters in elevation.
Haiti also has its share of mountain range chains. In the southwest is the Massif de la Hotte and Massif de la Selle in the southeast. The Massif de la Selle , with Pic la Selle that rises to 2674 meters, runs eastward to become the Sierra de Bahoruco. In the central area are the Montagnes du Trou-d’Eau and the Chaine des Matheux. In the north is the Massif du Nord and in the north center is the Montagnes Noires. In the northwest is the peninsula, Presqu’ile du Nord-Oest, a low ridge with arid areas.
The mean annual temperature is 24.40°C and the mean annual precipitation is 1438.40 mm. Located in the Caribbean’s Great Antilles, Haiti has a hot and humid tropical climate. Daily temperatures typically range between 19°C and 28°C in the winter and 23°C to 33°C during the summer months. Northern and windward slopes in the mountainous regions receive up to three times more precipitation than the leeward side. Annual precipitation in the mountains averages 1,200 mm, while the annual precipitation in the lowlands is as low as 550 mm. The Plaine du Gonaïves and the eastern part of the Plaine du Cul-de-Sac are the driest regions in the country. The wet season is long, particularly in the northern and southern regions of the island, with two pronounced peaks occurring between March and November.
Mean temperatures have increased by 0.45°C since 1960, with warming most rapid in the warmest season, June-November. The frequency of hot days and hot nights increased by 63 and 48 days per year, respectively, between 1960 and 2003. The frequency of cold days and cold nights has decreased steadily since 1960. Mean annual rainfall has decreased by 5 mm per month per decade since 1960. The intensity of Atlantic hurricanes has increased substantially since 1980.
The boas are found in primarily xeric areas, as opposed to the larger C.s. striatus that can be found in the less harsh deciduous environments. Schwartz secured an adult C. f. fordii from within the punky stem of an Agave while on a collecting trip in August, 1977 .
On the Dominican Republic the mean annual temperature is 23.73°C and the mean annual precipitation is 1448.18 mm.
The climate in the Valle de Neiba region on Hipaniola where C. fordii occurs is very arid and dry. The drier months are December, January, and February with less than 20mm rainfall, whereas the wettest moths are April and May and October with more than 60 mm precipitation .
Longevity
Chilabothrus fordii seem to live in general very long. The age record is held by a snake imported in 1972 as an adult wild caught animal, which lived until 2007. Making the possible age 50 years (pers. comm. Jay Wagner, Peter Tolson). More animals of methusaic age are known, such as one animal from a 1983 captive breeding from Joachim Bulian which is still alive in Germany (Hohmann pers. comm. 2016). S. Woodward is in possession of a female that was imported as an adult in 2003 and is still reproductively active (Woodward pers. comm.). Sinder and Bowler report a wild caught female kept at the Atlanta Zoo in Georgia, U.S.A. for 24 years and 5 months .
Previously it was assumed that smaller body size is negatively correlated to longevity . The data from captive specimens of C. fordii in comparison to its congeners casts doubt over a link between body size and long lifespan in the genus Chilabothrus. It would be interesting to have more data on longevity of reptiles at hand in general and from Chilabothrus particularly. Given that data on longevity are difficult to obtain in the wild, captive populations are a vital resource to study longevity and senescence and should be used to provide answers to study this important topic.
Reproduction
Mating normally takes place in December and January, the earliest of the genus. Eight gravid females collected on 8 May, 1977 from Thomazeau, Haiti, gave birth between 21 and 28 May. An additional female collected a day later from Petionville, Haiti gave birth three months later on 24 August . A female from Freres gave birth to seven neonates on 25 June. Another female taken at Petionville was gravid on 16 June . Neonate Chilabothrus are normally born between August and September, coinciding with the hatching of anoles and rainfall, to ensure a higher survival rate.

The first publication describing reproduction came from the Dallas Zoo (Murphy 1977) where a pair of C. fordii was kept in a terrarium 45 cm x 30 cm x 30 cm. The snakes were subadult when collected near Port-au-Prince, Haiti. Laboratory mice were offered weekly and accepted. Ambient temperatures varied between 27° C and 38° C relative humidity averaged 50%. Mating was observed 30th of January. The male coiled five complete loops around the female with two additional loops around midbody. After mating, the female continued to accept food until 6th of July. The birth of 5 live snakes occurred on the 20th of July. The newborns had a total length between 285 – 395 mm (mean 288 mm); snout-vent length 231-246 mm (mean 238 mm); weight 4.2-4.4 g (mean 4.3 g). The animals were kept together year round.

The same pair of adults gave birth to four young on 29 August 1975. Measurements and weights taken at birth are as follows: total length 24.6-25.7 mm (mean 25.1); SVL 19.6-20.7 mm (mean 15.2); weight 4.0-5.1 g (mean 4.4) .
In contrast to other Chilabothrus species, C. fordii mates earlier (December and January) whereas other species mate later in the breeding season; C. angulifer in May and June). Consequently the parturition follows earlier, even though several litters have been observed later in the year. Tolson data shows C. fordii often courting in late December or early January. Boas from different locales or populations may exhibit variations in the onset of courtship .
Timing of parturition in C. fordii based on reproductive events of captive bred and wild collected C. fordii shows a clustering of birthing events in summer. We used data from 43 birthing events from the following sources: and data collected by J. Murray and S. Woodward. Note that the first peak (End of May) is based on 8 birthing events from wild collected females from Thomazeau, Haiti and one captive breeding, whereas the other birthing events were mostly captive breeding events.
We analyzed data from 43 litters born in different years, different lineages, breeders from the EU, the U.S. and wild caught boas. Numbers of babies born ranged from 2-11. The mean litter size is 6.6 young, the median litter size is 7. Data from the following sources was used: J. Murray, S. Woodward (pers. comm.), .
The question about the best breeding strategy for this boa can’t be outlined with certainty. This boa species will reproduce using an “anything goes” approach. Successful breedings have occurred under the following conditions:
- animals were introduced as a group consisting of several males and females.
- a pair was kept together throughout the year.
- pair was kept separated and introduced during breeding season.
- In a group of 2.2 animals, the inferior male was kept with the females and the dominant male was separated throughout the year and only introduced during breeding.
Neonates of C. fordii belong to the smallest boa neonates. They weigh between 3.6 – 5.5 g, and are too small to consume even newborn mice. Newborn boas are best started on anole lizards and can be switched to newborn mice after several sheds. When started like this and fed regularly, C. fordii can reach sexual maturity within four years of age.

Behavior
Nocturnal and terrestrial, C. fordii is the sister taxon to C. gracilis. Because C. fordii evolved in sympatry with C. gracilis on Hispaniola, C. fordii it became entirely terrestrial and C. gracilis became entirely arboreal .

Ford’s boa is a small and crepuscular to nocturnally active boa. Like all members of the genus, Ford’s Boa is capable of secreting a strong (and not pleasant) smelling musk. The adults are a nervous when handled, however they usually don’t bite. Ford’s Boa is more terrestrial than several other species of the genus Chilabothrus; however, the boa has occasionally been found at night climbing in shrubs, trees and stonewalls, presumably, looking for sleeping anoles.
Diet
Diet in the wild consists of Anolis and small rodents (Mus) . Henderson et al. examined the stomach contents of 48 C. fordii. However, they found prey in only six of the specimens investigated. Thus, they concluded that the sample size is inadequate for detailed analysis. However, in the six prey items investigated were Anolis cybotes, Anolis sp. (4) and small rodents (Mus) (2). A boa taken at Manneville had a small rat in its stomach . These results – while important – show that we still lack knowledge about prey items in nature.
In captivity several strategies exist to get the young feeding. Jeff Murray starts the young on Anolis and found that they can be switched over to scented pink mice around the 12-15 month point. Michael Saina attempted to feed four newborn C. fordii with Mourning Geckos (Lepidodactylus lugubris). This prey offered in various sizes and on multiple occasions was not taken by the babies. Thus the boas were assist fed with baby mouse parts (legs and tails) and later on with hopper mouse tails. After 7-8 times of assist feeding, three out of four boas took small newborn mice (dead) voluntarily. The prey was offered in an clay flowerpot which was turned upside down and the whole in the bottom was a bit widened allowing easy access for the boa. The boas are extremely small when born, so any handling or assist feeding attempt needs to be performed with most possible care to avoid injuring the babies. The boas are at first too small to take baby mice as prey and therefore it is a matter of them being large enough to take the pink mouse as a food item. They grow very quickly once they feed voluntarily on rodents. Rodents taken in captivity are appropriately sized mice, pink rats, rat pups and baby quail. Males occasionally have to be “jump started” after breeding season by offering large anoles or chick fuzzed small mice to get them feeding again (Murray pers. exper.).
Captive management and captive population
This beautiful species does not require very large terraria and can be kept without great difficulties. The species is easy to keep once established and accustomed to their enclosure. Important to add are that newborn boas are so small that even the smallest baby mice appear too big for them. Thus having in the best case Anolis or another saurian food to raise these boas is mandatory. Even though Boas have been raised with assist feeding techniques where pieces of mice (tails or half mice) were fed , we consider saurian prey items beneficial for a problem free start into a long boa life. M. Saina noted that animals could be switched from anoles to mice using small live mice as prey items. The motility of the mice triggered the boas to prey upon the mice and later, dead mice would be taken as prey.


Currently (2000-2020) a self reproducing population of the nominate subspecies, Chilabothrus fordii fordii, is present in the private collections of American and European reptile keepers. Though not of general interest to the majority of private reptile keepers, several hobbyists enjoy working with this species. Therefore, the captive population of the nominate subspecies of Ford’s Boa seems stable at this time.

Conservation status, threats and population size in nature
CITES: Appendix II
Haiti is not a member of CITES.
Dominican Republic joined CITES on 17 December, 1986; entry into force on 17 March, 1987.
IUCN Red List: None
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
Nellis et al. reported in 1983 that Chilabothrus fordii occurs at high population density in some areas and question the dietary reasons for its success”. . 17 years later, Powell and Co-workers report that all three Dominican Boid species (Chilabothrus fordii, C. gracilis, C. striatus) “appear to be sustaining viable populations, sometimes even in heavily disturbed areas”. Despite this finding, they consider the protection status for the three species justified, because of their vulnerability to exploitation by the pet trade and, to a lesser degree, their dependence on threatened habitats . We would reverse these concerns as there is no pet trade regarding any of the West Indian Boas. These boas were last imported in 2003 in minute numbers.
The last import of boas from Hispaniola was in 2003; the pet trade is no longer a threat, and has not been for twenty years. It should not be a consideration of any consequence when determining population numbers or designing conservation measures. These snakes remain abundant in some areas, and the CITES listing reflects presumed threats emanating from international trade for the pet industry, which currently are not applicable to these species .
Powell and Incháustegui, two of the authors from the above study, report in a study published in 2009 that the introduced species, Bufo marinus has become widely distributed and is invading even very xeric areas that provide breeding sites only intermittently . While their study did not conclude to what extent Bufo marinus is affecting the Hispaniolan ophidian fauna, it can be estimated from the situation in Australia, where Bufo marinus was introduced as a Neozoon , that it will have a significant influence on population and conservation status of different snake species on the island of Hispaniola.
It should be also taken into consideration that the situation on Hispaniola, especially Haiti, has worsened for wildlife in general. The 2010 earthquake left Haiti largely ungoverned and the humanitarian crisis was followed by an ecological crisis due to destruction of habitats. Thus making it difficult to envisage a stable future for the snake species on the island. In addition to this, climate change is a factor which is likely to gain momentum in ecosystem changes throughout the West Indies. Giant storms fueled by rising oceanic temperatures as a consequence of human caused climate change are more likely to form more often than before. On the other hand, climate change will also increase droughts and diminish fresh water resources on the islands of the West Indies.
In a relatively recent study by Hedges et al. they predict, through modeling, that Haiti will experience mass extinction events by 2036 as a result of current deforestation trajectories. They comment: “We project that all primary forest in Haiti will disappear by ∼2035 (CI, 2033.5–2035.4) at the current rate, defining phase II as a 49-year period from 1986 to 2035 during which the final 4.8% of primary forest in the country will be lost. Assuming that our estimated loss of vertebrates is representative of the biodiversity in general, then 66–83% of species will be lost in Haiti during 1986–2035 because of deforestation. Thus, Haiti is well into a mass extinction, with only 8 of 50 mountains still holding primary forest. Unfortunately, Haiti’s neighbor, the Dominican Republic, is not a major refuge for Haitian species because more than half of the species surveyed (51%, average) on mountains with primary forest are endemic to Haiti and 12% are endemic to an individual mountain in Haiti. In addition, forest loss in the Dominican Republic is a threat to that country’s biodiversity.”
They continue: “It is common for deforestation to occur within protected areas of tropical countries, and, during the course of our surveys in Haiti, we observed ongoing destruction of primary forest inside all of the national parks. We also estimate that 60–75% of primary forest in the two original national parks, Pic Macaya and La Visite, has disappeared since they were declared as protected areas 35 years ago. In both cases, the rates of primary forest loss (pre-2000 and post-2000) were greater than the overall rates for all of Haiti, indicating that protection was minimal or nonexistent. This indicates that the mass extinction of biodiversity in Haiti will continue unabated unless greater protective measures are taken. More generally, this suggests that the phrase “protected area” be reserved only for areas where protection has been confirmed.” , .
A study on the risks for species extinction on island ecosystems worldwide analysed 15 insular regions, applying novel network analysis methods which enabled to disentangle the associations of multiple threats on vertebrates, invertebrates, and plants. The results showed that Biological invasions, wildlife exploitation, and cultivation, either alone or in association, are the three most important drivers of species extinction and decline on islands to date . These results are consistent with expectations when analyzing past and even current events. However, considering that climate change will dramatically change whole ecosystems in previously unprecedented ways, we estimate that this threat is more likely to affect species survival on an overall scale than the past drivers of extinction.
For a nation struggling to feed and house its’ people, conserving its fauna and herpetofauna are far from the top of the list, if they are on the list at all. Granted, there are measures in place, all on paper, that lay land aside for National Parks. Yet enforcing the measurements laid out on paper are anything but enforceable, as noted above. It is truly a travesty of the highest order; we can only hope some of the herpetofauna escapes the sickle of extinction.
Thus we consider the biggest threads for species survival in (1) large scale ecosystem changes, (2) invasive species, (3) climate change.
The CIA World Factbook lists the following environmental threats for Haiti: extensive deforestation (much of the remaining forested land is being cleared for agriculture and used as fuel); soil erosion; overpopulation leads to inadequate supplies of potable water and and a lack of sanitation; natural disasters. For the Dominican Republic: water shortages; soil eroding into the sea damages coral reefs; deforestation .
The map below is to illustrate the extent of habitat destruction and alteration due to development, intentional deforestation and agriculture.


On display in these Zoos
We are unaware of any live Chilabothrus fordii in Zoo or Academic collections anywhere in the world. If you have further information, please (Contact) us.
Fascinating Fords