Scientific Name
Chilabothrus strigilatus mccraniei

The subspecies was described and named by Sheplan & Schwartz. Bruce R. Sheplan was a herpetologist at Miami-Dade Community College. Albert Schwartz (1923 – 1992) was a Professor of Biology at Miami-Dade Community College. Both co-authored the seminal work "Hispaniolan boas of the genus Epicrates (Serpentes, Boidae) and their Antillean relationships."
Holotype
UMMZ: no. 118033. Subadult from Margaret Cay, Ragged Islands, Bahamas. Collected by Robert Hanlon on 7 September, 1957. Paratypes: ASFS V22851 on 16 November and ASFS V23468 on 17 November, 1970 by J.R. McCranie from Little Ragged Island.
Type Locality
Margaret Cay, Ragged Islands, Bahama Islands.
Synonyms
- Epicrates striatus mccraniei [84] [85]
- Epicrates striatus mccraniei
- Epicrates striatus mccraniei
- Epicrates striatus mccraniei : 56
- Epicrates striatus mccraniei
- Epicrates striatus mccraniei
- Epicrates striatus mccraniei : 21
- Epicrates striatus mccraniei Tipton, 2005: 46
- Epicrates striatus mccraniei : 104
- Chilabothrus strigilatus mccraniei
- Chilabothrus strigilatus mccraniei
- Chilabothrus strigilatus mccraniei : 15
- Chilabothrus strigilatus mccraniei : 282
Sheplan & Schwartz named C.s. mccraniei after J.R. McCranie, who collected the paratypes from Little Ragged Island on 16 and 17 November, 1970.
Common Name
Ragged Island Boa
Description and taxonomic notes
Chilabothrus strigilatus mccaniei was described together with C. s. ailurus, and C. s. fowleri by Sheplan and Schwartz based on a combination of various morphological characters . Their monumental work might be the most significant contribution to our understanding of the morphological characteristics of all Chilabothrus species, known at the time, and the variations of these species within the genus. At the time of their writing, the systematics of the Antillean forms were in a “chaotic state”. They examined living specimens of all Antillean taxa (at the time) except C. chysogaster and C. monensis. Click here to see their publication. We consider it a must read.
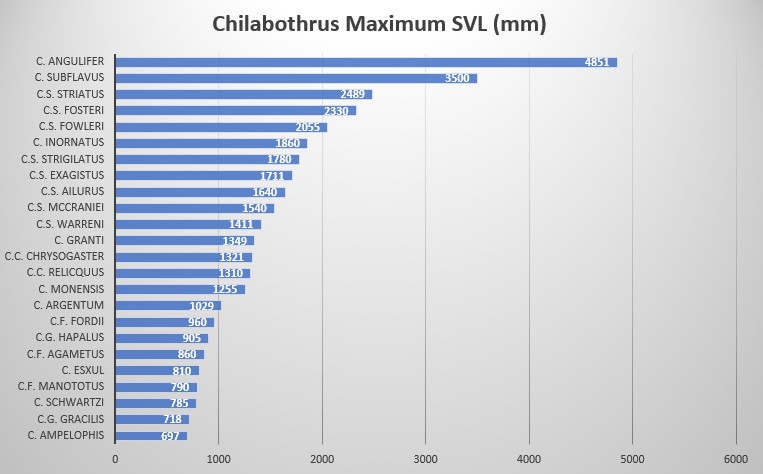
C. s. mccraniei is characterized by its small size: SVL of 1330 mm in males and 1540 mm SVL in females, low ventral counts of 270 in males and 266-271 in females, 2 intersupraocular scales (modally) anteriorly bordered by 3 scales (modally) and posteriorly by 4-6 scales (no mode), supralabials 14 (modally) with 2 scales entering the eye (7-8), 10-11 scales in circumorbital series (no mode) and 1 loreal (modally). The dorsal scale row formula is 41-50-26 .
C.s. mccraniei differs from all subspecies of striatus and strigilatus in that it has very dark coloration, extreme reduction of the lateral stripe in the neck, no dorsal blotch and 2 intersuprocular scales. It differs very much in color and pattern from exagistus and very much in color from C. s. warreni. C. s. mccraniei also has fewer ventrals and ventrals + subcaudals in both sexes. It differs from C. striatus (and C. s. strigilatus) by having 1 vs. 2 loreals, 14 vs. 15 supralabials, 7-8 supralabials entering the eye vs. 8-9.




Remarks: Chilabothrus strigilatus mccraniei was not mentioned in the following list of Caribbean Reptiles . We assume this was an inadvertent omission on the authors’ part and does not indicate a questioning of validity for this form.
Evolution
Fossil remains of Chilabothrus strigilatus have not been found to date. Subfossil remains from the late Holocene were found by Steadman and co-workers on Eleuthera . Reynolds et al. calculated based on molecular data that Chilabothrus strigilatus is on an isolated evolutionary trajectory since 2.6 MYA . Evolutionary research on the Ragged Island Boa lineage has not been undertaken to date.
Distribution
All subspecies of Chilabothrus strigilatus, are endemic to the Bahamas. The distribution of C. s. mccraniei is restricted to Ragged Island, Little Ragged Island, Margaret Cay.
[intergeo id=”AM2ETM”][/intergeo]
The five subspecies of Chilabothrus strigilatus occur on different islands of the Bahamas. The flags on the map indicate a type locality of a Chilabothrus strigilatus subspecies. Click on each flag to see which subspecies occurs on that particular island.

* Source
Habitat
In the Bahamas the mean annual temperature is 24.73°C (76.51° F) and the mean annual precipitation is 1264.46 mm. The islands experience warm, humid conditions year-round, though with more seasonal variations than the Southern Caribbean islands. There are also variations between the islands of the Bahamas, with rainfall falling twice as much in the northwestern islands than in the southeastern islands, and the more northerly islands experiencing temperatures up to 5° cooler than the southern islands.
Average temperatures are fairly high, with the mean daily temperatures fluctuating between 17°C and 32°C (62.6° F and 89.6° F). Mean annual rainfall for the Bahamas varies from about 865 mm to about 1470 mm. Inter-annual variability in climate is strongly influenced by the El Niño Southern Oscillation (ENSO). El Niño episodes bring warmer and drier conditions between June and August. Located in the heart of the Atlantic hurricane belt, the Bahamas is also subject to hurricanes and tropical cyclones especially during the August – November period.
Mean temperatures have increased by around 0.5°C since 1960, at an average rate of 0.11°C per decade. Bahamian data show that the mean daily maximum temperature for July has increased at a rate of 2°C per 100 years, and more recently at a rate of 2.6°C per 100 years. There have been statistically significant increases in the frequency of ‘hot’ days and nights, and decreases in ‘cold’ days and nights during the period 1973-2008.
There is seasonal variation in the rate of temperature increase, with the rate being most rapid in the warmest seasons, June-August and September-November, having rates of 0.13 and 0.15°C per decade respectively. There is also variation between islands, where the rate of warming is more rapid in the northeastern islands compared to the southwestern islands.
C.s. mccraniei was found in a rock wall near a well at dusk while extending its head from a hole. The following day another boa was found at the same wall in the same manner. Both became paratypes for the subspecies. The wall was surrounded by buttonwood swamp.

When asked for some context to the Ragged Island Boa photos, photographer Ed Casanno replied, “Not a very exciting story. At that time Louie (Porras) was operating The Shed in Miami and I was just starting my interest in reptile photography which took some equipment and skills compared to today. So when Louie would get in or catch something interesting he’d call up and say, “Ed, I’ve got something you need to take one more shot of.” That’s the way it was with that snake. I don’t remember who caught it, only that Randy McCranie was a mutual friend I had hunted with and of course we needed a photo of his namesake snake.”
Longevity
We assume C. s. mccraniei are as long lived like the other subspecies of C. strigilatus. We are not aware of any longevity records from captivity or wild specimens of this subspcies.
Behavior
Both paratype specimens were collected collected from a stone wall surrounding a buttonwood (Conocarpus) swamp at dusk. It is presumed that Chilabothrus strigilatus mccraniei are active boas, like the other subspecies. Unfortunately not much is known about this boa from the wild and records from captivity are missing as well. The low number of ventrals could indicate a more terrestrial lifestyle compared to other subspecies; however, this remains speculative for now.
Diet
No records on the diet of C. s. mccraniei have been published. We assume that – like the other subspecies of C. strigilatus – the neonates start on ectothermic prey such as Anolis and undergo a dietary change in later life stages.
Reproductive biology
While the reproduction of C. s. strigilatus, C. s. fosteri and C. s. fowleri has been studied in captivity and in the wild, we are not aware of any records or observations concerning the reproductive biology of C. s. mccraniei.
Conservation status, threats and population size in nature
CITES: Appendix II
Bahamas joined CITES on 18 September, 1976
IUCN Red List: Least Concern (LC)
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
At the time of this writing (Sept. 2019) IUCN lists the conservation status of C. strigilatus on a species level as least concern (LC). However, the conservation status of the subspecies on Ragged Island is unknown. This lack of data makes it impossible to determine if the population is in decline or even how large the population in total is. As for all Island fauna, the restricted range they inhabit is a constant threat for species survival. Considering the 2017 hurricane season in which hurricanes Irma and Maria, both category 5 storms, were passing through the region within two weeks of each other resulting in severe damages on many islands and leaving the Ragged Islands evacuated . One can hardly imagine the effect on the ecosystems. Human caused climate change will dramatically affect small island herpetofaunas in the coming years.
Data taken from Buckner et al. was used to generate the table below:
Taxon |
Number of islands |
combined total surface area in km2 |
| Chilabothrus s. strigilatus | 9 | 1548.57 |
| Chilabothrus s. ailurus | 2 | 390 |
| Chilabothrus s. fosteri | 4 | 17.86 |
| Chilabothrus s. fowleri | 4 | 6211.75 |
| Chilabothrus s. mccraniei | 2 | 4.42 |
C. s. mccraniei occurs on only two islands. The subspecies occurs in habitat with the smallest surface area of any C. strigilatus subspecies. Note that the above table displays the total surface area of the islands. This should not be falsely interpreted as suitable habitat. Detailed analyses about population size and suitable habitats are missing. We list this table to visualize potential threats as we consider it astonishing and worrying at the same time, that some subspecies occur on very small islands. It is worrying that the cane toad Rhinella marina has been recorded from New Providence as well as from Abaco Islands in the West Indies . Considering the devastating impact it had on the Australian herpetofauna, we hope that the West Indian species are less impacted by its occurrence.
The CIA World Factbook lists the following environmental threats for The Bahamas: coral reef decay; solid waste disposal
The map below illustrates the extent of habitat destruction and alteration due to development and agriculture.


To add an historic note to the map from 1737 is a passage from Catesby (1743) regarding the Bahamas iguana population and its role in the trade and food chain. He writes, “These Guana’s are a great Part of the Subsistance of the Inhabitants of the Bahama Islands, for which Purpose they visit many of the remote Kays and Islands in their Sloops to catch them, which they do by Dogs trained up for that Purpose, which are so dexterous as not often to kill them, which if they do, they serve only for present spending; if otherwise they sew up their Mouths to prevent their biting, and put them into the Hold of their Sloop till they have catched a sufficient Number, which they either carry alive for Sale to Carolina, or Salt and barrel up for the Use of their Families at Home. These Guana’s feed wholly on Vegetables and Fruit, particularly on a Kind of Fungus, growing at the Roots of Trees, and of this and others of the Anoma Kind.”
Population in captivity
We are not aware of any Ragged Island Boa being kept outside its natural habitat.
On display in these Zoos
We are unaware of this subspecies being kept in any zoological institution worldwide.

Citations