Scientific Name
Chilabothrus strigilatus fowleri

The subspecies was described and named by Sheplan & Schwartz. Bruce R. Sheplan was a herpetologist at Miami-Dade Community College. Albert Schwartz (1923 – 1992) was a Professor of Biology at Miami-Dade Community College. Both co-authored the seminal work "Hispaniolan boas of the genus Epicrates (Serpentes, Boidae) and their Antillean relationships."
Holotype
MCZ no. 125605 (originally ASFS V20366). Adult male from Fresh Creek, Andros Island, Bahamas. Collected by Danny C. Fowler on 7 September, 1970. Paratypes: AMNH 2923-24, 1908 by Dr. Dahlgren and Dr. Meuller; ASFS V15175 vic. Mangrove Cay, east shore, 3.2 km South airstrip on 25 April, 1968 by P.M. Baker; ASFS V20365 vic. 0.8 km South Fresh Creek on 5 September, 1970 by D.C. Fowler; ASFS V20461, 3.7 km South Stanniard Creek on 16 May, 1971 by A. Schwartz; ASFS V22303 from Chub Cay, spring of 1970, gifted by D.W. Thornton; ASFS V22304 from Great Harbour Cay on 5 May, 1970 by a local collector; MCZ 5819, no collector/date from Berry Islands, Bahama Islands.
Type Locality
Fresh Creek, Andros Island, Berry Islands.
Synonyms
- Epicrates striatus : 38 [39]
- Epicrates striatus fowleri [88] [89] [90]
- Epicrates striatus fowleri
- Epicrates striatus fowleri [14]
- Epicrates striatus fowleri
- Epicrates striatus fowleri
- Epicrates striatus fowleri : 21
- Epicrates striatus fowleri Tipton, 2005: 46
- Epicrates striatus fowleri : 96 [97] [98]
- Chilabothrus strigilatus cf. fosteri
- Chilabothrus strigilatus fowleri : 15
- Chilabothrus strigilatus fowleri : 282
- Chilabothrus strigilatus fowleri : 38
Sheplan & Schwartz named C.s. fowleri after Danny C. Fowler, who collected several specimens from Andros Island on 5 and 7 September, 1970.
Common Name
Andros Island Boa, Berry Island Boa.

Description and taxonomic notes
C. s. fowleri has been described by Sheplan and Schwartz based on a combination of various morphological characters . Their monumental work might be the most significant contribution to our understanding of the morphological characteristics of all Chilabothrus species, known at the time, and the variations of these species within the genus. At the time of their writing, the systematics of the Antillean forms were in a “chaotic state”. They examined living specimens of all Antillean taxa (at the time) except C. chysogaster and C. monensis. Click here to see their publication. We consider it a must read.
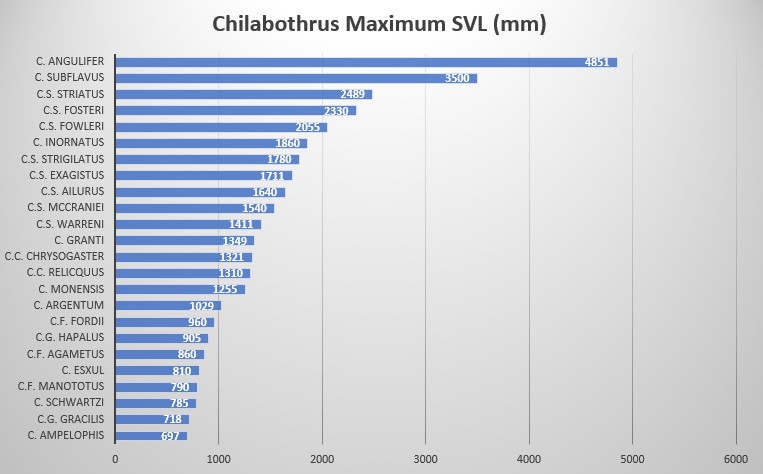
C. strigilatus fowleri is characterized by its large size: 1260 mm SVL in males and 2055 mm SVL in females, ventrals moderately numbered with 273-282 in males and 271-276 in females, intersupraocular 1 (modally), supralabials 15 (modally) with 2 scales (7-8) entering the eye, 10 scales (modally) in the circumorbital series and 1 loreal (always). Dorsal scale row formula is 40 – 51 – 27.
C.s. fowleri is a very dark boa with many fused dorsal blotching identifiable only by the pale remnant interspaces. Numbering between 78-112 on the body and 9-17 on the tail. The Berry Island boas can possess yellow chin and throat with the venter darker gray posteriorly. The neck stripe is normally short, breaking into secondary lateral blotching.
A recent molecular phylogeny came to conclude that the two subspecies C. s. fosteri and C. s. fowleri form a single clade. It was therefore suggested to treat C. s. fosteri and C. s. fowleri as a single form termed Chilabothrus strigilatus cf. fosteri . While the authors did a thorough analysis and used only specimens with confirmed origin, it should also be noted, that the sample size for the study was overall relatively small and in case of the Bimini and Berry Island boas consisted of a total of four specimens (1 Bimini Boa, 3 Berry Island Boas). While this analysis delivered results consistent in itself, we are cautious to follow their argumentation for several reasons and thus continue to treat the currently described subspecies of C. strigilatus as such. The traditional view of the subspecies was recently employed by the lead author of the study in another paper . Thus, until further research provides strong evidence to support the 2013 viewpoint, we consider all subspecies described so far as valid.
Evolution
Neither fossil, nor subfossil remains of Chilabothrus strigilatus fowleri have been found to date. Reynolds et al. calculated based on molecular data that Chilabothrus strigilatus is on an isolated evolutionary trajectory since 2.6 MYA .
Distribution
Andros Island, Chub Cay, Berry Island, Great Harbour Cay, Mangrove Cay and Sandy Cay. Sandy Cay is a range extension .
[intergeo id=”AM2ETM”][/intergeo]
The five subspecies of Chilabothrus strigilatus occur on different islands of the Bahamas. The flags on the map indicate a type locality of a Chilabothrus strigilatus subspecies. Click on each flag to see which subspecies occurs on that particular island.
C.s. fowleri: Sheplan and Schwartz (1974) identify a museum specimen from Mangrove Cay in Central Andros. Mangrove Cay lies between South Bight and Middle Bight; completely isolated from all other C.s. fowleri populations. This Mangrove Cay specimen is a female with a SVL of 980 mm and is, dorsally, a patternless medium brown. There is a blueish gray stripe that extends from the rear of the jaw to midbody. The boa is basically patternless but for some small darker brown blotches below the lateral stripe and a few scattered dorsal black scales. Since Central Andros and South Andros are both large land masses and completely separated from the east-west bight system, it is possible the boas separated from the boas on North Andros. This specimen is either an aberrant color/pattern of the subspecies or something new. More material from the Cay is needed to determine where this locale stands (Sheplan & Schwartz, 1974).
To our knowledge this has not been done.

* Source
Habitat
In the Bahamas the mean annual temperature is 24.73°C (76.51° F) and the mean annual precipitation is 1264.46 mm. The islands experience warm, humid conditions year-round, though with more seasonal variations than the Southern Caribbean islands. There are also variations between the islands of the Bahamas, with rainfall falling twice as much in the northwestern islands than in the southeastern islands, and the more northerly islands experiencing temperatures up to 5° cooler than the southern islands.
Average temperatures are fairly high, with the mean daily temperatures fluctuating between 17°C and 32°C (62.6° F and 89.6° F). Mean annual rainfall for the Bahamas varies from about 865 mm to about 1470 mm. Inter-annual variability in climate is strongly influenced by the El Niño Southern Oscillation (ENSO). El Niño episodes bring warmer and drier conditions between June and August. Located in the heart of the Atlantic hurricane belt, the Bahamas is also subject to hurricanes and tropical cyclones especially during the August – November period.
Mean temperatures have increased by around 0.5°C since 1960, at an average rate of 0.11°C per decade. Bahamian data show that the mean daily maximum temperature for July has increased at a rate of 2°C per 100 years, and more recently at a rate of 2.6°C per 100 years. There have been statistically significant increases in the frequency of ‘hot’ days and nights, and decreases in ‘cold’ days and nights during the period 1973-2008.
There is seasonal variation in the rate of temperature increase, with the rate being most rapid in the warmest seasons, June-August and September-November, having rates of 0.13 and 0.15°C per decade respectively. There is also variation between islands, where the rate of warming is more rapid in the northeastern islands compared to the southwestern islands.
Andros is not a single island, but rather a series of large islands separated by large tidal creeks or sounds. The three largest creeks are the North, Middle, and South Bights. Confusion results over the name Mangrove Cay, as it refers not only to one of the islands of Andros, but also to an extensive settlement on that island .
North Andros and South Andros differ quite significantly in habitat; North Andros has dense hardwood stands (coppice) while South Andros contains mostly pineland on limestone substrate. This difference might be explained by the geological and climatological history of the islands. A geo-climatological study on Blue holes in South Andros found that over the past 1500 years, South Andros shows evidence of four active periods of hurricane activity. None of these active intervals occurred in the past 163 years . While the consistency of the macro flora suggests relative geomorphic stability on South Andros over this time period, the hurricane frequency might have nonetheless limited reptile radiation and thriving on South Andros, due to severe conditions, lack of appropriate habitat and limitations in temperature and opportunities to bask. Thus, the comment of Sheplan & Schwartz on the lack of South Andros material is not entrely surprising. Even though the locals state the boas are found in the region . Further studies are needed to systematically analyze links between South vs. North Andros climate and reptile radiations.
Individuals of C. s. fowleri were found in a wide range of habitats, from xeric to mesic. In pine forest limestone walls, rotten logs, birds nests, clumps of vegetation on branches, in an oleander tree, in a boat hatch near the beach, in Coccoloba, near human settlements . On South Andros the boas can be found in broadleaf forest and beach scrub habitat with a mud and compacted lime ground surface. Owing to the diversity of habitats, we consider this species a generalist.
Longevity
Longevity records for this subspecies from wild or captivity are non existent. We assume that this subspecies is equally long lived as the other C. strigilatus subspecies
Behavior
C. s. fowleri are very active boas, and have been found climbing in various situations on Andros Island. C. s. fowleri was found in close vicinity of human settlements and human altered habitat , thus we consider the subspecies hemerophilous. C.s. fowleri have been shown to move great distances. On South Andros six boas were radio transmitter tracked between 19 August and 29 September 2003 using a hand-held 3-element Yagi directional antenna and a Wildlife Materials, Inc. TRX-48S receiver. The male boa from Sandy Cay traveled 1037 meters in 28 days; the Linder Cay boa traveled 462 meters in 15 days; the Mangrove Cay boas traveled 432 meters in 11 days, 793 meters in 5 days, 88 meters in 11 days and 215 meters in 10 days .
Diet
In the wild the young take small lizards (Anolis) as first foods, like the remainder of the genus (with the exception of C. angulifer). With growth the boas eventually shift their diet to rodents (introduced Mus and Rattus), birds, fowl and their eggs. A small C.s fosteri (ASFS V7121) contained a catbird (Dumetella) .
An interesting study from 2004, initiated quite by accident, was conducted when six C.s. fowleri consumed neonate Cyclura fitted with radio trackers. The opportunity was not lost on the researchers; the six boas were tracked, movements recorded and diurnal refugia sites were identified . This study documented the first incident of Cyclura predation by C.s. fowleri and Chilabothrus as a genus.
Three boas >80 cm consumed, from 3 separate clutches, 5 Cyclura hatchlings. One boa from Linder Cay consumed 3 hatchling Cyclura from the same clutch. A boa from Mangrove Cay consumed two Cyclura hatchings from the same clutch from several locations over a five night period. The rest of the boas consumed one Cyclura hatchling each. Transmitters were expelled from the boas between five and 28 days (Ibid).
In captivity the neonates start on ectothermic prey such as Anolis sagrei. It is possible that one or two from a litter might take a live pink mouse but they are the exception to the rule. Once the babies are feeding regularly they can be switched to pm by scenting them with anole skin, blood from the tail of an anole or chick fuzz. At this point the growing boas take appropriately sized rodents (rats and mice), chicks and quail.
Reproductive biology
Reports on the reproductive biology of C. s. fowleri are limited. Two adult female boas have been collected from Andros Island. The first was about 200 cm in length contained 26 eggs and the second had a SVL of 1509 and contained 35 eggs . In the past C. s. fowleri has been kept and bred by several breeders, including Brad Chambers and Tom Crutchfield, unfortunately no records of these breedings have been made public.
Captive management
Unfortunately to date, no information has been published on the keeping of C. s. fowleri, despite the fact that the subspecies has been kept in the US and Europe in small numbers. The standardized protocol for keeping and breeding West Indian Boas appears to be the most goal oriented way to maintain and breed C. s. fowleri.




Conservation status, threats and population size in nature
CITES: Appendix II
Bahamas joined CITES on 18 September, 1976
IUCN Red List: Least Concern (LC)
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
The Bahamas National Trust considers the boa to be endangered
At the time of this writing (Sept. 2019) IUCN lists the conservation status of C. strigilatus as least concern. This is however, based on the observation that because the number of mature individuals is decreasing they conclude that the population is decreasing as a whole. Unfortunately, a lack of data makes it impossible to say with certainty how steep the decline is, if any, and how big the population total is. As for all Island fauna, the restricted range they inhabit is a constant threat for species survival.
Data provided by Buckner et al. was used to generate the table below:
Taxon |
Number of islands |
combined total surface area in km2 |
| Chilabothrus s. strigilatus | 9 | 1548.57 |
| Chilabothrus s. ailurus | 2 | 390 |
| Chilabothrus s. fosteri | 4 | 17.86 |
| Chilabothrus s. fowleri | 4 | 6211.75 |
| Chilabothrus s. mccraniei | 2 | 4.42 |
The total surface area of the islands as outlined here should not be falsely interpreted as suitable habitat. Detailed analyses about population size and suitable habitats are missing. We list this table to visualize potential threats as we consider it astonishing and worrying at the same time, that some subspecies formed on very small islands. It is worrying that the cane toad Rhinella marina has been recorded from New Providence as well as from Abaco Islands in the West Indies . Considering the devastating impact it had on the Australian herpetofauna, we hope that the West Indian species are less impacted by its occurrence.
The CIA World Factbook lists the following environmental threats for The Bahamas: coral reef decay; solid waste disposal
The maps below illustrate the extent of habitat destruction and alteration due to development and agriculture.






To add an historic note to the map from 1737 is a passage from Catesby (1743) regarding the Bahamas iguana population and its role in the trade and food chain. He writes, “These Guana’s are a great Part of the Subsistance of the Inhabitants of the Bahama Islands, for which Purpose they visit many of the remote Kays and Islands in their Sloops to catch them, which they do by Dogs trained up for that Purpose, which are so dexterous as not often to kill them, which if they do, they serve only for present spending; if otherwise they sew up their Mouths to prevent their biting, and put them into the Hold of their Sloop till they have catched a sufficient Number, which they either carry alive for Sale to Carolina, or Salt and barrel up for the Use of their Families at Home. These Guana’s feed wholly on Vegetables and Fruit, particularly on a Kind of Fungus, growing at the Roots of Trees, and of this and others of the Anoma Kind.”
Population in captivity
Very few specimens of breeding size exist in collections in the US. The subspecies was previously also kept in Europe, however we are unaware of any specimens in collections in Europe at the moment of writing.
On display in these Zoos
We are unaware that this species is kept in any zoological institution worldwide
Fascinating Fowleri

Citations