Scientific Name
Chilabothrus striatus exagistus

The subspecies was described and named by Sheplan & Schwartz. Bruce R. Sheplan was a herpetologist at Miami-Dade Community College. Albert Schwartz (1923 – 1992) was a Professor of Biology at Miami-Dade Community College. Both co-authored the seminal work "Hispaniolan boas of the genus Epicrates (Serpentes, Boidae) and their Antillean relationships."
Holotype
MCZ no. 125603 (originally ASFS X3780) from vicinity Camp Perrin, Dpt. de Sud, Haiti. Adult female, one of a series, captured by local collectors on 24-26 July, 1962.
Paratypes ASFS X3779 and ASFS X3781-88 same data as holotype; USNM 60603, Moline 28 January, 1918 by W.L. Abbott and USNM 60604, Les Basses 9 January, 1918 by W.L. Abbott, Ile-a-Vache, Haiti; ASFS V9678 Jeremie by a local collector on 18 March, 1966; ASFA X3789-91 by local collectors on 6 August, 1962.
Type locality
Camp Perrin, Departement du Sud, Haiti.
Synonyms
- Epicrates striatus exagistus [73] [74]
- Epicrates striatus exagistus
- Epicrates striatus exagistus
- Epicrates striatus exagistus
- Epicrates striatus exagistus Henderson, Schwartz & Inchaustegui, 1984: 94
- Epicrates striatus exagistus [46] [47]
- Epicrates striatus exagistus
- Epicrates striatus exagistus : 21
- Epicrates striatus exagistus Crother, Powell et al, 1999: 114
- Epicrates striatus exagistus Tipton, 2005: 46
- Epicrates straitus exagistus : 132 [134]
- Chilabothrus striatus exagistus Reynolds et al, 2013:
- Chilabothrus striatus exagistus : 14
- Chilabothrus striatus exagistus : 38
The name striatus is derived from the Latin for "striated" or "lined". The name exagistus is from the Greek for "mystical". A reference to voodoo in that the snakes are associated with the Loa Damball (Letburn 1966).
Common Name
Tiburon Boa
Description and taxonomic notes
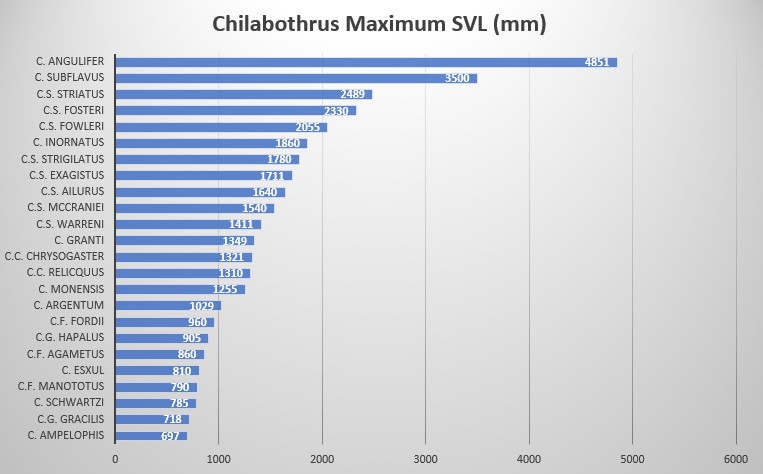
Chilabothrus striatus exagistus is a moderately sized boa with males to 1711 mm SVL and 1635 mm SVL for females, ventrals 271-285 in males and 273-286 in females. It is characterized by 2 intersupraocular scales (modally), bordered anteriorly by 6 scales and posteriorly by 5 scales (modally), supralabials 15 with one or two supralabials entering the eye (8 or 7-8), 10 or 11 scales in the circumorbital series (modally) and 1 loreal (modally).
In most specimens the dorsum is virtually patternless to faintly patterned with a unicolor tan. It lacks the pale dorsal median spots or dots on the neck. C.s. exagistus differs from C. striatus in that:
- It has a faded pattern and pale coloration.
- It is smaller in size.
- The head scale formula of 6-2-5 is different from the C.s. striatus formula of 5-2-5.
- The modality of supralabials entering the eye is 8 or 7-8 vs. 8-9 in C.s. striatus.
- It has a very high modality of a single loreal.
- it is separated from C.s. warreni by the lower number of ventrals + subcaudals.
Note that the live boa presented above does not display the faded coloration that was used as a characteristic in the subspecies description. Multiple explanations might be possible. Most likely is the time the museum specimen(s) spent in preservative. However, these will all remain speculative without a thorough study of the Tiburon Boa.
The holotype of Chilabothrus striatus exagistus. Photo MCZ



* Source
The holotype of Chilabothrus striatus exagistus. Photo MCZ



Evolution
A molecular phylogeny based on CYTB locus found that Chilabothrus striatus is most closely related to C. exsul, C. schwartzi and C. argentum: the lineages split about 2.2 Mya. The next closest relative to the clade is C. strigilatus, from which the lineages split 2.6 Mya. Then there is C. chrysogaster, from which the lineages split 4.1 Mya .
Distribution
Hispaniola: Haiti, distal western portion of the Tiburon Peninsula and Ile-a-Vache. East to Les Basses. From sea level to about 305 meters . Franz and Cordier suppose that this subspecies probably occurs farther east as they report that intergrades between C. s. exagistus and C. s. striatus exist near Jacmel . Unfortunately they don’t describe the snakes from this region in detail.

* Source
[intergeo id=”wM1ETM”][/intergeo]
Three subspecies of Chilabothrus striatus occur on Hispaniola. The flags on the map indicate the type locality of a subspecies. Scroll over to see which subspecies has its type locality at the indicated point.
Habitat
On Haiti the mean annual temperature is 24.40°C and the mean annual precipitation is 1438.40 mm. Located in the Caribbean’s Great Antilles, Haiti has a hot and humid tropical climate. Daily temperatures typically range between 19°C and 28°C in the winter and 23°C to 33°C during the summer months. Northern and windward slopes in the mountainous regions receive up to three times more precipitation than the leeward side. Annual precipitation in the mountains averages 1,200 mm, while the annual precipitation in the lowlands is as low as 550 mm. The Plaine du Gonaïves and the eastern part of the Plaine du Cul-de-Sac are the driest regions in the country. The wet season is long, particularly in the northern and southern regions of the island, with two pronounced peaks occurring between March and November.
Mean temperatures have increased by 0.45°C since 1960, with warming most rapid in the warmest season, June-November. The frequency of hot days and hot nights increased by 63 and 48 days per year, respectively, between 1960 and 2003. The frequency of cold days and cold nights has decreased steadily since 1960. Mean annual rainfall has decreased by 5 mm per month per decade since 1960. The intensity of Atlantic hurricanes has increased substantially since 1980.
C.s. exagistus occurs in Ile-a-Vache; Jeremie from sea level to 305 meters (vic. type locality). Possibly at a higher elevation (Moline) in the northern foothills of the Massif de la Hotte. Like E. s. striatus, exagistus is primarily a snake of mesic wooded situations; one
specimen was secured by Richard Thomas in woods at night as it crossed a paved road .
Longevity
No longevity record for C. s. exagistus exists. C. s. striatus in comparison are long lived. The age record for C. s. striatus confirms an age of 6 years and 8 months at the National Zoological Park . J. Wagner (pers. comm.) is in posession of an old male from one of the authors that was an adult when imported in the early 2000’s and is still alive. Bowler listed the maximum age for an Epicrates striatus ssp. at 22 years and one month . While we can not exclude with certainty that this record refers to C. striatus, we assume that this record refers to a Chilabothrus strigilatus ssp., since no Chilabothrus striatus warreni and very few C. s. exagistus have been exported and kept.
Reproduction
Sheplan & Schwartz collected seven gravid females vicinity Camp Perrin from 24 to 26 July. All females had seven to eighteen fetuses in them. While SVL of the females varied from 1420 mm to 1635 mm, there was no correlation between litter size and SVL. Two of the smallest females had the upper limit for fetuses and one of the largest females had but seven embryos . We are unaware of this subspecies ever being bred in captivity.
Behavior
Given the subspecies close proximity to the nominate species, it can be assumed its behavior is highly likely to parallel that of the nominate species. C. striatus is nocturnal and mostly arboreal in nature. Smaller boas have been observed crawling through vegetation at night while, presumably, searching for sleeping Anolis. Large boas have been observed at night loosely coiled on branches of low trees. Diurnal behavior includes resting on large branches, in the shade, at heights of five to twenty meters off the ground. They can be also be found in limestone crevices, hollow trees, and bird nests .
Diet
Henderson et al. examined 133 C. striatus and found 28 prey items. The boas go through an ontogenetic shift in prey items as they mature. Boas that measure less than 60 cm SVL preyed primarily on Anolis. Boas measuring 60 to 80 cm SVL preyed on both Anolis and small rodents. Those boas larger than 80 cm SVL preyed on birds and rats (Rattus rattus). Once boas’ measurements exceeded 105 cm SVL their diet consisted entirely of rats. In 1974 an extremely large C. striatus (248.9 cm total length) was found that contained remains of a Plagiodontia in its digestive tract (C.A. Woods, reported in Ottenwalder, 1985). Bats are preyed upon, but only where they are localized geographically. The prey items of the 133 boas examined consisted of:
- 2.1% lizards (Anolis coelestinus, A. cybotes, A. sp)
- 5.2% birds (Dulus dominicus, Quiscalus niger, Coereba flaveola(?))
- 92.7% mammels (Rattus rattus, Mus musculus)
It was determined the mean size of anoles taken (including C. fordii and C. gracilis), to be 3.6 cm³ (2.5 to 4.6 cm³). C. striatus preyed upon the larger anoles (of a species) more so than any other boid species. They posit the large boas feed only upon large prey because the prey is both ecologically and geographically abundant and widespread .
Captive management
We can’t say if the husbandry requirements differ from the nominate subspecies. Given that the Tiburon peninsula differs in climate from other parts of Hispaniola might indicate slight adjustments in humidity levels and possibly temperature compared to the nominate species. We assume the boas will thrive if given ample space, several places to hide and branches to climb and rest upon. A separate soaking container is recommended, in addition to a water bowl. Dietary requirements are most likely similar to the nominate species.
Conservation status, threats and population size in nature
CITES: Appendix II
Haiti is not a member of CITES.
IUCN Red List: Least Concern (LC)
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
These snakes remain abundant in some areas, and the CITES listing reflects presumed threats emanating from international trade for the pet industry, which currently are not applicable to these species . The island of Hispaniola is politically divided from East to West in two sovereign states: Dominican Republic and Haiti. Two of the three recognized subspecies occur only on the Haitian side (Chilabothrus striatus exagistus and C. s. warreni) of the island, whereas the nominate subspecies C. s. striatus occurs in different habitats spread across the whole island. Haiti became one of the poorest countries in the world from its once flourishing state. Haiti suffers from high degrees of poverty; as a result of this Haiti is largely deforested. The conservation efforts undertaken in the previous years have largely fallen victim to inactivity by the fractured departments within the unstable government. Travel to and within Haiti is considered high risk. The recent earthquake and hurricane tragedies have compounded all of Haiti’s problems.
The CIA World Factbook lists the following environmental threats for Haiti: extensive deforestation (much of the remaining forested land is being cleared for agriculture and used as fuel); soil erosion; overpopulation leads to inadequate supplies of potable water and and a lack of sanitation; natural disasters .
The map below illustrates the extent of habitat destruction and alteration due to development and agriculture.



Population in captivity
A small population of the nominate species, C.s. striatus, is present in the private collections of American and European reptile keepers. We are unaware of any C.s. exagistus in any Public Institutions or private collections worldwide.
Anecdotal information
Doris M. Cochran wrote, in 1924, of Dr. W.L. Abbott:
“For the past thirty-five years Dr. W. L. Abbott has enriched the collections in the United States-National Museum by frequent contributions of the results of his collecting expeditions in various parts of the world. Since 1916 he has turned his attention particularly to the island of Haiti, from which he has sent much valuable material,
including many new or rare species of animals and plants. During the summer and autumn of 1916 Doctor Abbott collected natural history specimens on the Samana Peninsula in northeastern Santo Domingo. This trip proved so beneficial to the needs of the National Museum that Doctor Abbott has returned to the island each year. His second trip was made during the first six months of 1917 when he secured many specimens from Tortuga Island and from the northern and northwestern parts of the Republic of Haiti. In
November of the same year he made a third trip, this time covering southwestern Haiti and Cayemites Island. From February to October, 1919, he visited the Samaria Peninsula once more, and worked to the southwest toward Duverge. In the spring and early summer of 1920, Doctor Abbott visited Gonaives Island and some small villages in the vicinity of Furey, Haiti. The three expeditions taken since that time have all been to the Samana Peninsula, from which district very rich collections have been secured where formerly few specimens had been obtained” .