Scientific Name
Chilabothrus gracilis hapalus

The subspecies was described and named by Sheplan & Schwartz. Bruce R. Sheplan was a herpetologist at Miami-Dade Community College. Albert Schwartz (1923 – 1992) was a Professor of Biology at Miami-Dade Community College.
Holotype
MCZ no. 125602. Adult male from Camp Perrin, Department du Sud, Haiti. One from a series collected by native collectors on 28 July, 1962.
Paratypes: Haiti: ASFS X3243-49, X3251, same data as holotype; ASFS X2805-06, same locality as holotype, July 24, 1962, D. C. Leber, native collectors; MCZ 64781, Mayette, near Jeremie, Dept, du Sud, December 12, 1960, L. Whiteman; ASFS V25005, 11.2 km W Jeremie, Dept, du Sud, June 19, 1971, R. Thomas; MCZ 65095-97, Ca Ira, Dept, de l’Ouest, March 15, 1961, L. Whiteman; MCZ 116757-59, Leogclne, Dept, de l’Ouest, spring, 1969, collector unknown; USNM 117277, Diquini, Dept, de l’Ouest, March 17, 1943, A. Curtiss; USNM 123987, Diquini, Dept, de l’Ouest, November 23, 1946, A. Curtiss; USNM 118035, near Port-au-Prince, Dept, de l’Ouest, August 1943, A. Curtiss.
Additional specimens: Dominican Republic, Barahona Province: 5.3 km NE La Cienaga (ASFS V30814); 7.7 km W Parafso, 153 meters (ASFS V30952-53, ASFS V31029) .
Type Locality
Camp Perrin, Departement du Sud, Haiti.
Synonyms
- Epicrates gracilis hapalus [118] [119] [120]
- Epicrates gracilis hapalus
- Epicrates gracilis hapalus
- Epicrates gracilis hapalus Henderson & Schwartz, 1984: 45
- Epicrates gracilis hapalus Henderson, Schwartz & Inchaustegui, 1984: 93
- Epicrates gracilis hapalus [46] [58]
- Epicrates gracilis hapalus
- Epicrates gracilis hapalus : 21
- Epicrates gracilis hapalus Crother, Powell et al, 1999: 114
- Epicrates gracilis hapalus Tipton, 2005: 45
- Epicrates gracilis hapalus : 132
- Chilabothrus gracilis hapalus : 12
- Chilabothrus gracilis hapalus : 38
The name hapalus is derived from the Greek for "gentle, delicate", in reference to the boa's nature.
Common Name
Tiburon Vine Boa.
Description and taxonomic notes
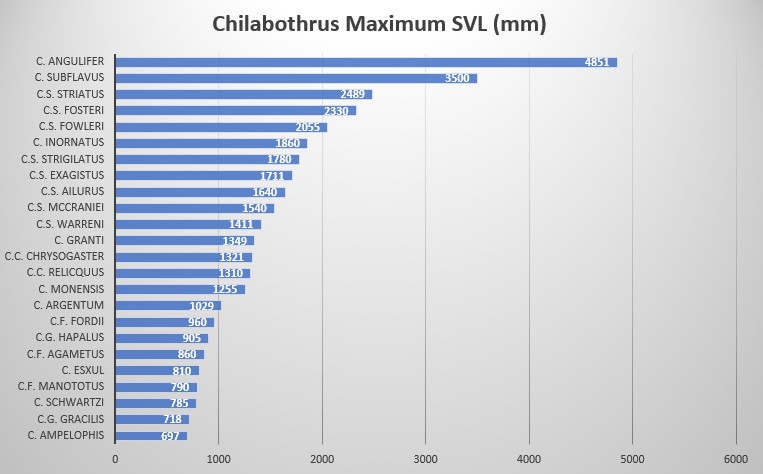
C.f. hapalus is characterized by its large size: SVL in males is 870 mm and 905 mm in females, high ventral count in both sexes, males are 278-304 and 279-296 in females, high subcaudal counts of 96-111 in males and 90-100 in females, ventrals + subcaudals are also high at 376- 415 in males and 373-396 in females, 13 suprlabials, 13 infralabials, preoculars 1 and 3, postoculars 6, circumorbital scales 11 and 12, 1 intersupraocular scale bordered anteriorly by 3 scales and posteriorly by 2 scales, 6-7 and 6-8 supralabials entering the eye; dorsal scale row formula is 35 – 44 – 26.
While there is no obvious color or pattern difference between C.g. hapalus and C.g. gracilis, there are several characteristics that separate the two subspecies. They are:
- C.g. hapalus has neck and middorsal scale counts of 31 – 42 and 34 – 47; C.g. gracilis has 27 – 38 and 33 – 42.
- Ventral scale in counts in males of both subspecies are 278-304 ( 290.6 mean) in C.g. hapalus and 271-286 (278.6 mean) in C.g. gracilis.
- Ventral scale counts in females are 279-296 (286.8 mean) for C.g. hapalus and 271-286 (278.7 mean) for C.g. gracilis.
- Ventral + subcaudal scale counts for male C.g. hapalus are 376-415 (395.6 mean) and 368-377 (362.6 mean) for C.g. gracilis.
- Ventral + subcaudal scale counts for female C.g. hapalus are 373-396 (383.3 mean) and 364-383 (375.3 mean) for C.g. gracilis.

According to data provided by museum specimens, male and female C. gracilis reach a SVL of 700 mm and 718 mm, respectively. In C.g. hapalus the difference in size is very pronounced, with males reaching 870 mm while females reach 905 mm. This large sample size indicates C.g. hapalus is much larger that the nominate species, C.g. gracilis .

Remarks: A population of C. gracilis exists at the east side of the Barahona Peninsula in the south of the Dominican Republic. Sheplan and Schwartz examined four specimens from this population and noted that they were similar in size to the C. g. hapalus specimens, however the ventral counts of these snakes resembled closer those of C. g. gracilis. Thus Sheplan and Schwartz suggested that this population represents extreme intergrades between C. g. hapalus and C. g. gracilis. They noted that there are no specimens of C. g. hapalus closer than 150 kilometers to the west (Port-au-Prince, Diquini, Ca Ira) in Haiti, although apparently suitable habitat occurs abundantly between the eastern coast of the Peninsula de Barahona and the base of the Tiburon Peninsula . However, Tolson and Henderson, as well as Henderson and Powell assigned this population with a question mark . It is to this day unclear if this population is a natural variation of C. g. hapalus, an intergrade form between the two subspecies or a third - yet undescribed - subspecies of C. gracilis.



* Source
Distribution
Tiburon Peninsula east to Port-au-Prince and Jacmel, Haiti and the Peninsula de Barahona in the Dominican Republic. Almost all localities of C.g. hapalus are coastal or nearly coastal. Found from sea level to about 305 meters .
[intergeo id=”AOzETM”][/intergeo]
The flags on the map indicate the type locality of Chilabothrus gracilis and Chilabothrus gracilis hapalus. Scroll over the flag to see which type locality occurs at the indicated point.
Habitat
On Haiti the mean annual temperature is 24.40°C and the mean annual precipitation is 1438.40 mm. Located in the Caribbean’s Great Antilles, Haiti has a hot and humid tropical climate. Daily temperatures typically range between 19°C and 28°C in the winter and 23°C to 33°C during the summer months. Northern and windward slopes in the mountainous regions receive up to three times more precipitation than the leeward side. Annual precipitation in the mountains averages 1,200 mm while the annual precipitation in the lowlands is as low as 550 mm. The Plaine du Gonaïves and the eastern part of the Plaine du Cul-de-Sac are the driest regions in the country. The wet season is long, particularly in the northern and southern regions of the island, with two pronounced peaks occurring between March and November.
Mean temperatures have increased by 0.45°C since 1960, with warming most rapid in the warmest season, June-November. The frequency of hot days and hot nights increased by 63 and 48 days per year, respectively, between 1960 and 2003. The frequency of cold days and cold nights has decreased steadily since 1960.
Mean annual rainfall has decreased by 5 mm per month per decade since 1960. The intensity of Atlantic hurricanes has increased substantially since 1980.
Chilabothrus g. hapalus is nocturnal and arboreal. The Tiburon Peninsula, particularly at its end is mostly mesic. All the boas taken from La Cienaga and Paraiso were found in mesic habitat. It is assumed habitat for C.g hapalus does not differ much from the habitat of the nominate species .
On the Dominican Republic the mean annual temperature is 23.73°C and the mean annual precipitation is 1448.18 mm.
Longevity
We are unaware of any publications documenting the longevity of Vine Boas. The oldest living specimen we are aware of is a captive bred specimen produced from adults that were imported as adults in 2003. (J. Wagner pers. comm.)
Reproduction & reproductive triggers
Tolson reported on two wild caught boas that gave birth to three live babies and 2 unfertilized ovum each on 21 and 24 October. The babies weighed between 2 g and 2.3 g with relative litter masses of 0.192 and 0.224 . Neonate SVL is 300 mm . A further wild caught female caught at Limbe, Haiti gave birth to 4 babies on 22 September, 1978. .
Behavior
C. gracilis is the sister taxon to C. fordii. Because C. gracilis evolved in sympatry with C. fordii on Hispaniola it became entirely arboreal and C. fordii became entirely terrestrial .
Diet
The diet of C. g. hapalus has not been studied. We assume it is similar to the nominate subspecies as dietary specialist preying on anoles. Henderson et al. examined the stomach contents of 33 C. gracilis. Unfortunately the publication does not discriminate between subspecies and neither could we find references which museum specimens were examined to trace back the location of capture. The boas examined preyed solely on Anolis cybotes and A. distichus .
Captive management
Even though no records or anecdotes about the care of the Tiburon Vine Boa have been published, we assume it requires similar care as the nominate subspecies. This means a spacious enclosure with plenty of branches and plant material attached for them to hide in during the day. An elevated water bowl will enable them to find the water. Because they are nocturnal all feeding should be done after lights out. These boas are most likely prey specialists that can not survive for a longer time period on a diet of rodents.
Conservation status, threats and population size in nature
CITES: Appendix II
Haiti is not a member of CITES.
Dominican Republic joined CITES on 17 December, 1986; entry into force on 17 March, 1987.
IUCN Red List: None
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
These snakes remain abundant in some areas, and the CITES listing reflects presumed threats emanating from international trade for the pet industry, which currently are not applicable to these species. Commonly found in suitable habitat and with little commercial appeal, C. gracilis is considered stable in number. Alteration of its habitat and loss of prey items are the current threats facing this slender oddity. Few photos exist of both subspecies of gracilis; in particular the rare C. g. hapalus. The Tiburon Vine Boa pictured below was killed by a local.

It is unfortunate the only photo of C.g. hapalus in situ is of a dead specimen. Reith comments on the photo above: “[And no] I have no idea how that snake died… [Well] they are often killed by local people who fear the devil …”.
Habitat destruction and deforestation in particular is one of the major threats to wildlife in Haiti. The demand for charcoal (for local consumption as well as export) dramatically deteriorated the situation. Loss of primary forest is a major driver of mass extinction events, particularly in areas with a high degree of endemism such as Haiti . The alteration of habitat is of particular concern for the survival of C. g. gracilis and C. g. hapalus considering that these boas are more adapted to mesic situations and nowhere very common .
The CIA World Factbook lists the following environmental threats for Haiti: extensive deforestation (much of the remaining forested land is being cleared for agriculture and used as fuel); soil erosion; overpopulation leads to inadequate supplies of potable water and and a lack of sanitation; natural disasters .
The map below illustrates the extent of habitat destruction and alteration due to development and agriculture.


Population in captivity
We are unaware of any C. gracilis hapalus in private or public collections in the past or present. CITES trade records indicate that the species C. gracilis was imported several times, however the entries in the cites trade database do not discriminate between subspecies. Hence, it is possible that these imports contained also C. g. hapalus. We could not trace down any of the imported boas.
On display in these Zoos
We are unaware of any Chilabothrus gracilis hapalus on display in any public institutions or zoo collections worldwide.