Scientific Name
Chilabothrus ampelophis

Described and named by Miguel A. Landestoy T. of the Universidad Autónoma de Santo Domingo, Dominican Republic, R. Graham Reynolds of the University of North Carolina Asheville, USA, and Robert W. Henderson of the Milwaukee Public Museum, USA.
Holotype
Museo Nacional de Historia Natural Prof. Eugenio de Jesús Marcano MNHNSD 23.3901. Paratypes: MNHNSD 23.3900; MNHNSD 23.3902; University of Kansas Biodiversity Institute (KUH 352337); Museum of Comparative Zoology, Harvard University (MCZ R-197400).
Type Locality
Hispaniola, Barahona Peninsula, near the Dominico-Haitian border.
Subspecies
None.
Synonyms
Chilabothrus ampelophis Landestoy T., Reynolds & Henderson, 2021: 6
The epithet originates from ancient greek ampelos, meaning vine, in allusion to the slender body and head shape, and for the relative abundance of vines in the dry rocky habitat at the type locality. The suffix -ophis refers to a snake, hence the epithet is translated as "vinesnake".
Common Name
Hispaniolan Vineboa
Description and taxonomic notes
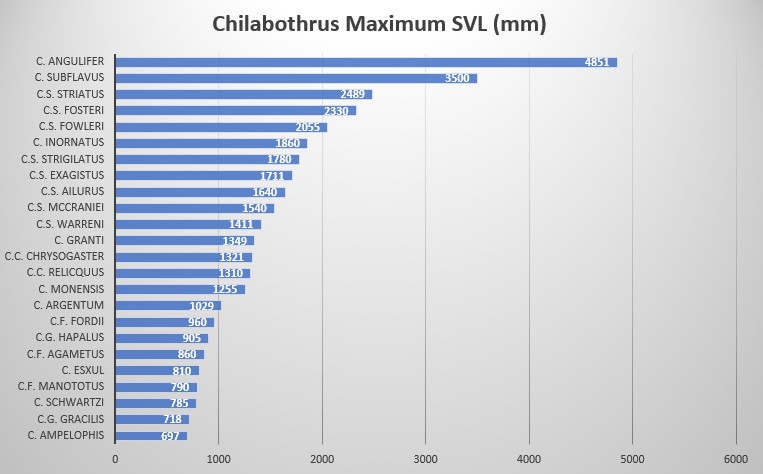
According to the original description, based on five specimens, the boa is a small arboreal species of Chilabothrus with a slender body, characterized by a maximum SVL of 697 mm in females and 629 mm in males. Detailed meristics, see table below. The authors suggest that given the close proximity to its sister species C. fordii, it might be inferred C. ampelophis is sexually dimorphic. The dorsal coloration is described as a dark taupe/tan-brown with a dorsally connected/sometimes broken pattern of dark brown to black scales that are one-three scales wide at the intersections and bends. The pattern gives an impression of a broken lattice pattern using rectangles, Xs and odd squares.
Landestoy et al. describe the coloration and patterning as follows: dorsally tan-brown grading into taupe to pale gray on the sides. Several scales are stippled with dark brown. The dorsal primary pattern elements form a series of very dark (dark brown to black) narrow diagonal blotches that are mostly fused, creating a zigzag effect. Also some isolated irregular X-shaped, squarish, and rectangular blotches are present, all bordered with whitish cream scales; two paramedian longitudinal dark-brown to blackish stripes on the neck departing from the occiput to ~11 scales back, each of a width of three to four scales, edged by a thin (one-scale wide) nearly continuous pale cream line. A lateral dark-brown stripe that originates at the head (evident to midbody but reappears posteriorly) and repeatedly and irregularly branches toward dorsolateral and lateroventral areas, forming somewhat diffuse reticulated markings. Venter pale cream to taupe patterned with brown to dark-brown stippling and freckling, and peripheral smudges on ventral scales that gradually increase in occurrence and density posteriorly; subcaudals mostly dark brown (from subcaudal 13 to tip of tail) especially at the center.

Head Pattern. The basic head pattern is consistent in the series. Dorsal surface of head: dark brown overall with taupe suffusions on the interorbital and prefrontal areas, having the center of the parietal area with a faint pale cream bilobed figure bisected posteriorly, followed by six diffuse pale cream spots arranged transversely with two anteriorly and four posteriorly in the occipital region, each encircled by darkbrown to blackish scales, and pale cream lobes entering sides of parietal areas from the temporal region (from postocular pale Y-shaped stripe, see below). Lateral head pattern: two horizontal Y-shaped stripes, one of which is a postocular pale cream stripe and the other is a preocular dark brown stripe. The former is rather asymmetrical, with upper lobe shorter, extending briefly to the dorsal surface of the head, bordered below by a dark brown stripe that descends posteriorly (and connected but not aligned to the lateral body stripe) to the base of the posteriormost supralabial scale. It reappears as a preocular Y-shaped dark brown stripe bisected (disrupted on the right).

This species displays the following morphological characteristics: Length of the five specimens analyzed ranged from 447–776 mm; average of 664.8 mm.
Distribution
The species has been found in a single, very small area of less than 10 km2 along the Dominican-Haitian border on the southern slopes of the Sierra de Bahoruco on the Barahona Peninsula. All specimens were discovered at altitudes between 80 – 105 meters above sea level . Given the near total deforestation on the Haiti side near this location, it is entirely possible this boa was once found on the Haitian side of the border and was extirpated some time in the past.
[intergeo id=”gDO5QTM”][/intergeo]
The map shows the approximate locality of C. ampelophis
Important Note
While discussing the newly described species, Alfred Delmonte (personal comm. 2021) informed us that he, together with Marcellino Hernadez, were on a research trip to Beata Island southwest of the Barahona Peninsula in 1999. Here they collected a boa which was – based on the memories of A. Delmonte – very similar to C. ampelophis. The snake was brought back alive and handed over to Professor Sixto Incheaustégui (Dominican Republic). However, through a series of unfortunate events, the snake died while Professor Inchéaustegui was absent from the office and once he returned, the snake was in a stage of decomposition that prevented any further analysis or description. If the snake was indeed a member of the species C. ampelophis the distributional range of C. ampelophis extends about 60 km south from the original range presumed by Landestoy T. et al. If the snake was a different form, we might see more species descriptions in the future.
Habitat
The region where C. ampelophis was found is composed of karstic limestone of the Barahona Peninsula and the foothills of the southern Sierra de Bahoruco. The vegetation shifts from mesic along the sides of the Río Pedernales to more xeric in the neighboring karst foothills.

Longevity
unknown
Reproduction
unknown
Behavior & Diet
Landestoy et al. described an interesting behavior in two of their study objects. While being photographed, the tails and parts of the posterior portions of the bodies of two of the boas became rigidly straight while the posterior portion of the snakes’ body was hanging off a branch or even while the snakes moved and slid away in a stealthy manner. They assume this behavior may have the purpose of balancing or crypsis. Regarding foraging behavior and diet of C. ampelophis, the scientists presume that it likely parallels that of the similarly slender and highly arboreal C. gracilis. They consider it likely to be an active forager that hunts for quiescent prey, primarily Anolis lizards, at night. Further studies are needed to investigate the ecology and behavior of this boa.
Conservation status, threats and population size in nature
CITES: Status pending
Dominican Republic joined CITES on 17 December, 1986; entry into force on 17 March, 1987.
Haiti is not a member of CITES.
Red List: pending
Tree of Life Catalog: pending
The National Center for Biotechnology Information: (click here)
CITES Import/Export Data:
The finding that other threatened species, such as Peltophryne armata, Anolis strahmi, Sphaerodactylus plummeri, Sphaerodactylus thompsoni, an undescribed Tropidophis, and Mitophis pyrites are found in the habitat, indicates a degree of still functioning ecosystem. However, the authors anticipate that the habitat of this new species is directly threatened from resource exploitation activities, and thus urge additional work to further characterize the conservation status of the species.
Landestoy T. et al., have suggested a listing of Critically Endangered by IUCN based upon the assumption that the species is micro-endemic and highly localized, since no individuals of this species were previously found in adjacent habitats. Given the complete deforestation of Haiti near this locale, it is not inconceivable the boas once existed there and were extirpated as a result of the deforestation. Further exploration of the surrounding area should determine the outer boundaries of the new boa.
The authors of the study point out the habitat lies along the Dominico-Haitian border and remains quite well forested. However, they saw intensive agriculture in the neighboring lowlands and wood charcoal production is happening directly at the type locality. Furthermore, habitat alteration (removal of small trees and bushes of Amyris spp.,
which is exported overseas for the perfume industry), is occurring locally. Free-roaming cattle may negatively affect the habitat. As a result, the cattle destroy or alter prey habitat that has a direct impact on the boas ability to find or feed upon preferred prey items .
We fully agree with the risk analysis provided, and support the claims for listing based on current knowledge. However, it will be interesting to see if the species is indeed a microendemic or, if it occurs on other parts of the Barahona Peninsula (See: Important Note above). We are somewhat astonished to see that the researchers did not consider or even mention human caused climate change as a direct threat. Especially considering that it will change the face of the West Indies drastically and it is likely a severe threat to island herpetofauna. Hurricane intensification and increase in numbers of storms are the certain effects of climate change droughts, erosion and others factors will follow suit .
The CIA World Factbook lists the following environmental threats for Haiti: extensive deforestation (much of the remaining forested land is being cleared for agriculture and used as fuel); soil erosion; overpopulation leads to inadequate supplies of potable water and and a lack of sanitation; natural disasters. For the Dominican Republic: water shortages; soil eroding into the sea damages coral reefs; deforestation .
The map below is to illustrate the extent of habitat destruction and alteration due to development, intentional deforestation and agriculture.


Population ex-situ
Per Landestoy (pers. comm.), an adult pair of C. amelophis has been provided to Zoo Dominica. It is with foresight these boas enter a program like the hugely successful C. granti and C. monensis SSPs. It is our recommendation a group of adult boas be secured for study in country and at equally successful Zoos in the US. We know literally nothing about the ecology of this new boa. A captive population would therefore help to determine its ecology, morphology and reproductive biology.
Questions such as:
- When does reproduction take place?
- How long is gestation?
- How many neonates constitute an average litter?
- What do the neonates weigh, what is their SVL?
- What do the neonates look like, what color(s) are they?
- What do the neonates feed on for first foods?
- Do the offspring undergo an ontogenetic color change like many other species in the genus? If so, how long does it take to complete?
- How long does it take to reach sexual maturity? At what age are the males and females considered mature? Is there a different age for each sex?
- How long lived are the adult boas?
Only by answering these questions will we understand the life cycle of this amazing new boa. This knowledge, in turn, would help to determine conservation measures and a thriving ex-situ population can function as a safety backup population. With few exceptions, much of what we know and understand about this entire genus has been through captive breeding programs in both the public and private sectors.
The press releases for this new boa are here and here.



