Scientific Name
Chilabothrus angulifer

Described and named by Dr. Jean Theodore Cocteau (1798-1838), a famous scientist and physician and Gabriel Bibron (1806-1848), a herpetologist and zoologist at the Museum National d'Histoire Naturelle, Paris.

The first scientific image of a Cuban boa Chilabothrus angulifer. This image was drawn by french artist Jean Gabriel Pretre and appeared in Volume VIII of the monumental work covering all aspects of Cuba by Ramon De La Sagra. Volume VIII appeared in 1855 and contains the folio collection with illustrations of all species described in the previous volumes. The original description of the Cuban Boa by Cocteau and Bibron appeared in 1843 in volume IV.

Chilabothrus angulifer. Description, in Latin, from the original description reads “corpore supra fulvo an albicante maculis angulosis fuscis ornato” and translates to “above the body is decorated with yellow or whitish brown angular spots”.
Henderson and Barreto assume that “The specific name originates from the Latin word angirlus, meaning “angle,” probably in reference to the angular shapes of the main elements of the dorsal pattern.” . Photo Ryan Potts
Holotype
Muséum national d’Histoire Naturelle: Paris, no. 3292.
Type Locality
Cuba.
Subspecies
None.
Synonyms
- Boa…. Gundlach, 1840: 361
- Epicrates angulifer de la Sagra, 1843: 129 [130] *
- Epicrates angulifer : 215 [216] [217]
- Epicrates angulifer 560 [561] [562]
- Epicrates angulifer Gray, 1849: 94 [95]
- Epicrares angulifer de la Sagra, 1855: tab XXV
- Epicrates angulifer Hinrich Lichenstein, 1856: 23
- Epicrates angulifer Jan, 1863: 24
- Epicrates angulifer Jan, 1864: pl VI
- Epicrates angulifer Jan, 1865: 89
- Epicrates angulifer Gundlach, 1875: 362
- Epicrates angulifer : 70 [71] [72] [73] [74]
- Epicrates angulifer Boulenger, 1893: 96
- Epicrates angulifer [61] [62]
- Epicrates angulifer angulifer : 5
- Epicrates angulifer Werner, 1901: 37
- Epicrates angulifer
- Epicrates angulifer : 326
- Epicrates angulifer Ragues, 1914: 29 [Ragues or Raques?]
- Epicrates angulifer : 304
- Epicrates angulifer : 186 [187] [188]
- Epicrates angulifer
- Epicrates angulifer : 140
- Epicrates angulifer : 107
- Epicrates angulifer
- Epicrates angulifer : 131
- Epicrates angulifer
- Epicrates angulifer : 149
- Epicrates angulifer angulifer : 74
- Epicrates angulifer : 44
- Epicrates angulifer angulifer : 188 [189]
- Epicrates angulifer
- Epicrates angulifer : 60 [62] [63] [64] [65] [66]
- Epicrates angulifer
- Epicrates angulifer
- Epicrates angulifer : 84
- Epicrates angulifer
- Epicrates angulifer
- Epicrates angulifer : 7
- Epicrates angulifer : 14
- Epicrates angulifer Liner, 1994: 22
- Epicrates angulifer : 19
- Epicrates angulifer Crother, et al, 1999: 51 [61]
- Epicrates angulifer
- Epicrates angulifer : 9
- Epicrates angulifer Tipton, 2005: 43
- Epicrates angulifer : 116 [117] [118] [119] [120] [121] [122] [123] [124]
- Epicrates angulifer Gower et al, 2012: 61
- Chilabothrus angulifer **
- Epicrates angulifer
- Chilabothrus angulifer : 9
- Chilabothrus angulifer Murphy & Crutchfield, 2019: 180 [181]
- Chilabothrus angulifer : 37
- Chilabothrus angulifer
* Epicrates is from the Greek Επικρατης meaning “powerful.”
** Chilabothrus is from the Greek meaning cheilos “lip”, á “without”, and bothros “pits”.
Common name
Worldwide known as Cuban Boa. On the Island of Cuba known as Majá de Santamaría which is best translated as: Beautiful Girl (or Beauty, Beauty queen) of the holy Mary.
Taxonomic history
Chilabothrus angulifer was described as Epicrates angulifer in the 4th Volume of Ramón de la Sagra’s work about Cuba. De la Sagra’s history is worth a publication by itself. In short, Spanish born, living in Paris, de la Sagra appears to be a naturalist par excellence. During his travels to Cuba, he collected specimens and after returning to Paris wrote a monumental 12 volume work entitled: Histoire Physique, Politique et Naturelle de I’Ile de Cuba. The time span during which this work was published ranged from 1838 –1857 (French edition) and 1838 –1861 (Spanish edition). He was assisted by many prominent French scientists at the time who wrote chapters or volumes of the book, though de la Sagra oversaw and edited the entire 12 volume series. Cocteau and Bibron assisted him by writing the reptile section. Even though Cocteau died prematurely in 1938, he authored, post mortem, the Reptiles chapter together with Bibron. The 4th volume was published in 1843 (not 1840 as often stated) and contained the description of Chilabothrus angulifer. Both, the French and the Spanish edition appeared in 1843. The plate (tab XXV) depicting the species for the first time appeared in volume 8, published in 1855. This volume was a folio collection of all species described in the previous volumes and contains spectacular illustrations worthy of framing and hanging on one’s wall.
Description and taxonomic notes
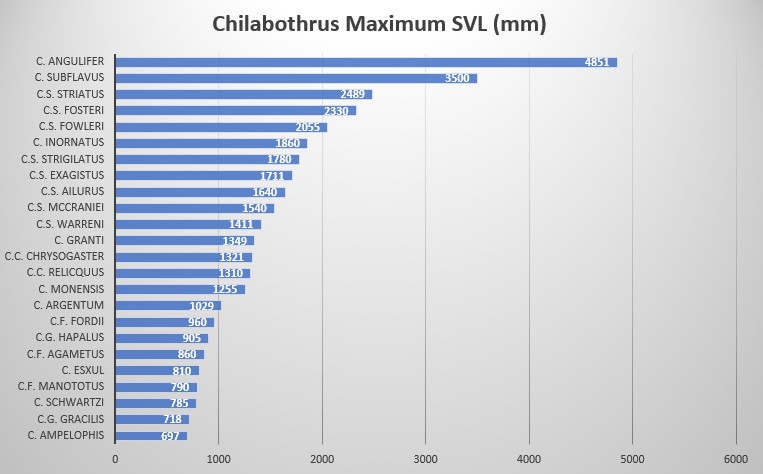
The presence of labial pits, the shortest tail (45-55) of the entire genus (66-93) and the supralabials separated from the eye result in the Cuban Boa being the least derived species of the genus Chilabothrus. These three characteristics are only shared by the mainland Epicrates and Eunectes. Chilabothrus angulifer is also the largest species of the Genus Chilabothrus. Maximum size has been described by Gundlach (cited by Barbour and Ramsden 1919) to reach seven yards ( 6.40 meters)! In 1989, sadly, a 15 foot 11 inch (4851 mm) female was run over and killed by a truck on Guantanamo Naval Base on Cuba. She had the reproductive ability to produce 30 offspring every two years .


Gundlach (1880) records specimens from the Cienaga of seven yards and had one himself of five yards, which is probably the one now stuffed in the Museo Gundlach. A specimen of almost this size was received some years ago alive at the New-York Zoological Park and was a most extraordinarily bulky reptile. A living Boa about nine feet long was caught in the Cienaga in 1915 and two others in 1913 of about the same size (Barbour). Examples over ten feet long are certainly rare today, although the junior author has seen a skin over twelve feet long with head and neck cut off.
While a total length of 6.40 meters appears extraordinarily large, Barbour and Ramsden mention several other specimens reaching well above 4 meters in length . Contrary to this, Sheplan and Schwartz investigated 55 animals covering different locations within the species boundaries. The longest males reached 1.74 meters SVL and the largest female 2.25 meters SVL. Interestingly, they found that snakes from the Isla de la Juventud were considerably smaller, reaching greatest length of 1.56 meters for females and 1.48 meters for males. . Similar island-mainland differences in size (and other morphometric characters) have been described for Boa constrictor by .

The pholidosis and other characteristics of Chilabothrus angulifer are as follows: maximum SVL is 4000 mm , 272-292 ventral scales in males, 268-290 ventral scales in females. 45-55 subcaudal scales in males, 46-54 subcaudal scales in females. Ventrals + subcaudals 321-347 in males, ventrals + subcaudals 316-339 in females. Intersupraocular scales modally 3, pre-intersupraocular scales modally 3, post-intersupraocular scales modally 4; dorsal scale row on neck 44-54, 53-69 at midbody, 28-38 anterior to vent, the lowermost scale not alternating a large and small scale for each ventral scute; supralabials modally 14 with none entering the eye in most instances, the supralabials and eye separated by a complete row of lorilabials; infralabials modally 17; loreal modally 1; circumorbital series modally composed of 8 scales; dorsal pattern consisting of from 42 to about 65 appressed angulate dark-brown to black markings on a yellowish to yellowish-tan ground, often (western Cuba) w/out any dark colors in the dorsal pattern, the pattern composed of an indeterminate number of medium-brown to pale-tan, much fused markings; tail patternless above, or with up to 12 dorsal darker markings . Grant noted differences in shade between the north and south coasts of Oriente Cuba. His description is based on two specimens only found on Banes and Guantanamo respectively. The two males appeared similar in size (4ft, 6in.) and squamation, however noted that the upper right 8th labial enters orbit in the southern specimen. He noted that the background coloration of the northern specimen is distinctly brown in contrast to gray of the south .
* Taken from
While no subspecies of Chilabothrus angulifer have been described so far, two different color forms exist, which occur in different localities on eastern and western Cuba . Cuba is divided from East to West in two different ecoregions. The West belongs to the summer wet tropical climate zone, whereas the East of the island is part of the permanently wet tropical climate zone . Thus it is possible that the different color forms, on an east-west cline, are adaptations to different ecological pressures and could represent ecomorphs of the same species. More research is necessary to determine the answer to this and several other important questions.




Interestingly, Sheplan and Schwartz found that snakes from the Isla de la Juventud were considerably smaller than individuals from the rest of the distributional range, reaching a maximum total length of 1.56 meters for females and 1.48 meters for males , whereas specimens from other sites are often much larger. While size is not a valid standalone criterion for the description of subspecies, it might function as a first criterion. Thus it will be interesting to see if other morphological characters differ between populations as well.
Sheplan and Schwartz comment: “Nonetheless, we are reluctant to name a new subspecies of this widely-ranging snake until more material is available. It is of interest that the plate in the original description shows a pale and faded snake, so there is little question that the nominate form, should a new subspecies be named, will be the western pale population” .
A similar analysis was conducted on populations of Boa constrictor located on the islands of the coast of Belize compared to mainland populations . Further studies using molecular data suggested that the genus may represent multiple species . In this light it will be interesting to see if the species Chilabothrus angulifer, might in fact comprise several different species or subspecies.
The most recent study on the cuban boa by Rehák an co-workers analyzed the mitochondrial haplotype structure of the European ex situ population of Cuban boas. The results revealed a high degree of diversity. 96 specimens were sequenced and 25 distinct haplotypes detected. The results further indicated a deep divergence among three principal haplogroups, with bayesian estimates of the divergence time equal to 3.57 and 2.26 Mya respectively. Rehák and colleagues consider this divergence as an argument for diverse evolutionary lines whose distance corresponds to or is greater than among some other – taxonomically recognized – species of the genus . Whether these lines represent whole species or subspecies needs to be tested by further in depth studies with genetic material collected from different locations in cuba.
Distribution
Restricted to the island of Cuba, Isla de la Pinos, Isla de la Juventud, other offshore satellite islands and cays, such as Cayo Cantiles in the Archipielago de los Canarreos, Archipielago de los Colorados and Archipielago de Sabana-Camagüey. Widespread both geographically and altitudinally from sea level to 1214 meters .
The type locality is pictured in the center of the map.
[intergeo id=”QN3ATM”][/intergeo]
Localities: Pinar del Río province: Luis Lazo; Las Acostas (Brattstrom 1958); Viñales (Černy 1966); 2 km W Bolondrón; 18 km W Manuel Lazo (Garrido and Schwartz 1968); cueva del Agua, Guanahacabibes (Dacal and Pino 1968); La Bajada; María La Gorda (Černy 1969); San Vicente; 4 km San Vicente; Ancón valley; San Diego de los Baños (Sheplan and Schwartz 1974); Tierra Buena, 24 km W La Bajada (Godínez et al. 1987); cueva de La Barca; cueva de Perjuicio; cueva de la Ventana, 8 km S Valle de San Juan (Armas et al. 1989); Mil Cumbres (Alfonso et al. 1998); San Ubaldo-Sabanalamar (MP8). La Habana province: Havana city (Pérez Vigueras 1934); América vicinity; Calabazar; cueva de La Santa, Bacuranao; Tarará (Varona and Arredondo de La Mata 1979); Alturas del Cacahual (Crespo and Jiménez 2004); Rincón de Guanabo (MP24); Atabey; Parque Zoológico Nacional (CZACC). Artemisa province: Guanajay (Barbour 1914); Rancho Mundito (Neill and Allen 1957); Guajaibón (Zajicek and Mauri 1969); cueva de Paredones, San Antonio de los Baños (Varona and Arredondo de La Mata 1979); Galalón, Sierra del Rosario (Berovides and Carbonell 1998); cueva de Los Portales (Rodríguez Gómez et al. 2005); Soroa; El Salón; El Rubí; Nortey (Rodríguez Schettino et al. 2005 b); El Mulo; Mariel (MNHNCU). Mayabeque province: cueva del Mudo, Catalina de Güines; cueva del Cura, Escaleras de Jaruco (Černy 1969); cueva del Indio, Tapaste (Silva Taboada 1974); Boca de Jaruco; 10.9 km E Jaruco; 3.2 km E Boca de Jaruco (Sheplan and Schwartz 1974); cueva del Túnel, 3 km SE La Salud (Acevedo et al. 1975). Matanzas province: Península de Hicacos (Buide 1966); Santo Tomás (Černy 1966); cueva de Ambrosio (Silva Taboada 1974); Canal del Arroz, Los Hondones (Sampedro and Montañez 1989); Bermejas (Estrada 1995); Los Sábalos (Amorín et al. 2003); Jicarita (Rodríguez Schettino et al. 2005a); Soplillar; cayos Blancos del Sur (Rodríguez Schettino and Rivalta González 2007); Caverna de Santa Catalina (MP19); cayos de las Cinco Leguas (MP25). Villa Clara province: Jibacoa (Schwartz and Ogren 1956); cayo Santa María (Garrido and Jaume 1984); El Salto del Hanabanilla; cayo Las Brujas; cayo Ensenachos; Cubanacán (Alfonso et al. 1998); Mogotes de Jumagua (Morell et al. 1998); Monte Ramonal (MP11); Las Loras (MP20); Santa Clara (USNM). Cienfuegos province: Guajimico; Jardín Botánico de Soledad (Schwartz and Ogren 1956); cueva de Guajimico (Silva Taboada 1988); Yaguanabo (Rodríguez Schettino et al. 2005a); Guanaroca-Punta Gavilán (MP6). Sancti Spiritus province: Topes de Collantes; Finca Morales; cueva de Caguanes (Sheplan and Schwartz 1974); dolina de Los Cocos, Trinidad (Varona and Arredondo de La Mata 1979); El Jíbaro (Garrido 1980c); Jobo Rosado (Alfonso et al. 1998); Tunas de Zaza (MP2); Lebrije (MP12); Lomas de Fomento (MP13); Alturas de 69 Banao MP16); cueva de los Majáes, Trinidad (USNM). Ciego de Ávila province: río Majana, Majagua; 13.4 km Morón (Sheplan and Schwartz 1974); Cayo Coco (Regalado 1981); cayo Paredón Grande (Martínez Reyes et al. 2005); La Maya; Chambas (Rodríguez Schettino et al. 2005a); Loma de Cunagua (MP22). Camagüey province: Senado (Cochran 1934); cueva del Rancho, Limones (Brattstrom 1958); 8.8 km N Banao; Los Paredones, Sierra de Cubitas; Paso de La Trinchera; Paso de Lesca (Sheplan and Schwartz 1974); cayo Guajaba (Schwartz and Henderson 1988); cayo Romano (Martínez Reyes et al. 2005); cayo Sabinal; Lugareño (Rodríguez Schettino et al. 2005a); cueva de Rolando (Díaz 2006); cueva Gaspar-Najasa; cueva Rosa La Bayamesa; cueva Dos Majáes (Rodríguez Schettino and Rivalta González 2008); Río Máximo (MP4). Las Tunas province: ró Tana (MP7). Granma province: Leonero dam (Montañez et al. 1985); Arroyo Colorado, Guisa (Berovides and Carbonell 1998); Cabo Cruz (Estrada and Ruibal 1999); El Macío (MP26); Pico Caracas (MP27); Bartolomé Masó sugar cane factory (CZACC); Bayamo (AMNH). Santiago de Cuba province: Santiago de Cuba (Alayo 1951); 3 km S, 11 km NE Sevilla; La Gran Piedra; 35.8 km W Santiago de Cuba (Sheplan and Schwartz 1974); cueva en Cativar, El Cobre; cueva de los Majáes, Siboney (Silva Taboada 1974); cueva de los murciélagos, La Uvita (Silva Taboada 1988); El Gato (Viña and Armas 1988); El Indio, Parque Baconao (Álvarez and Milián 1997); Reserva Ecológica Siboney (Fong and del Castillo 1997); La Mesa, La Bayamesa (Díaz, Fong, Viña and Knell 2005); Loma del Gato; El Ramón de las Yaguas; Alto Songo (CZACC). Holguín province: Banes (Grant 1960); arroyo El Palo, 5 km SE Mayarí (Varona and Arredondo de La Mata 1979); Cayo Saetía (Estrada 1992); El Toldo (Estrada 1996); cayos de la Bahía de Sagua de Tánamo (Fernández et al. 2000); La Melba; Cupeyal del Norte (Fong, Díaz and Viña 2005); Pico Cristal (MP14); La Mansura-Pilotos (MP15). Guantánamo province: Baracoa (Barbour 1914); cueva de los Bichos; Maisí (Barbour and Ramsden 1919); US Naval Base, Guantánamo (Grant 1960); 24 km Sabanilla; Mabujabo (Sheplan and Schwartz 1974); río Toa; Yunque de Baracoa; Alto de Iberia; Pico Galán (Estrada 1996); Hatibonico littoral (Díaz 2002); Bahía de Taco (Borroto-Páez et al. 2002); Ojito de Agua (Fong, Díaz and Viña 2005); Yara-Majayara (MP28); Potosí (CZACC); 3 mi W Baitiquirí (USNM). Isla de la Juventud: Punta del Este; cayo Cantiles; cayo Largo del Sur (Černy 1969); Sierra de Casas; Los Indios; 14.1 SSW Nueva Gerona (Sheplan and Schwartz 1974); cayo Ávalos; cayo El Rosario (Estrada 1993a); Punta Francés (MP29); sierra de la Cañada (MP30); Guayacanal (CZACC); Pedernales (MNHNCU); río Soldado (LRS). (Schettino, Mancina, Gonzalez, 2013).
Habitat
On Cuba the mean annual temperature is 25.27°C with average minimum of 10°C and a maximum average of 35°C. The mean annual precipitation is 1342.37 mm with most areas receiving from 1100 mm to 1600 mm. A few areas receive as little as 300 mm while others receive as much as 3000 mm in a year. The climate is tropical with a dry and relatively cool season from late November to mid-April, and a rainy season from late April to early November. In the south, where the only mountainous areas are found, there is a greater difference between the north-facing slopes, which are very wet, and those exposed to the south, where precipitation drops below 700 mm (27.5 inches) per year .
The island has three main regions: the highlands, the plains and the foothills. The plains make up 66% of the island. There are four main mountain regions: the expansive Sagua-Baracoa massif, the Guaniguanico system, the Sierra Maestra system and the Guamuhaya system. The Pico Real is the highest point in Cuba at 1974 meters and is within the Sierra Maestra system. Cuba is made up of lowland, submontane, montane, swamp, mangrove and pine forest. Deforestation has been taking place to allow for cattle and sugar-cane plantations. In 1812 approximately 89% of the island was forested. That dropped to 54% in 1900, 14% in 1959 and slightly increased to 18% in 1987 . CEO magazine reports Cuba was 30.67% forested in 2019 (data here).

C. angulifer is found in woodlands at both low and high elevations. It has been found as high as 9.2 meters above the ground in trees. Inhabits bunkers on Guantanamo (U.S. Naval Base), crossing roads at night, shrubbery on roadsides, rocky cliffs and hillsides, rocks at river cuts, entrances to caves and in caves, grassy slopes and grassy hillsides .

Longevity
In the wild the boas can live in excess of 30 years. Although the reproductive potential is still poorly understood, long term studies are quantifying the missing or inconclusive data. In captivity the animals continuously reproduced at the age of 30 plus years .
Reproduction & reproductive triggers
C. angulifer females are biennial breeders and take five or more years to mature. Mating season is normally April though Jun. Males will mate every year and engage in ritualized combat; courtship is necessary for follicular development and ovulation. Size, not age, determines the female’s ability to reproduce. Gestation in the wild is typically 150-180 days . Gestation length appears to reflect the temperatures the females are exposed to while gravid. Parturition normally takes place in September and October. Litter sizes range from 2-22 young in the wild. There appears to be a correlation between the size of the female and litter size/neonate size; the larger the female the larger the litters and babies . Neonate SVL is 540 mm -618 mm . The young are born the largest of any boa in the genus and are capable of taking mice and rats after the first shed. The babies’ first shed takes place approximately 3 weeks after birth.
Rodriguez-Cabrera et al have determined, through a field study, the minimum reproductive size and age requirements for C. angulifer in the wild. The study confirmed earlier findings be Tolson and Teubner that reproductive maturity is determined by size and not age. Several breedings between different sized males and females were recorded; data such as SVL of both sexes, litter size, neonate size and weight were all taken. Using capture and recapture data, they were able to determine monthly and annual growth rates of the boas. The study also confirmed that the boas are still killed – without reason – by humans on the island.
In the first breeding a male boa (measuring 1272 mm SVL, 158 mm tail length and 900 g) was captured. As it was out in the daytime and it was considered breeding season, they assumed it was in search of a female. This male was placed with a captive born female almost 8 years old (2233 mm SVL, 217 mm tail length and 9525 g). After repeated copulations the female went through a gestation period of 169-177 days. In October she gave birth to 13 neonates (593-646 mm SVL, 53-67 mm tail length and 140-157 g).
In April of 2013 a breeding group of nine boas (one female and eight males) was discovered inside a hollow log in second-growth forest. Unfortunately the local person who discovered them also killed them. The researchers were told of the location, where they examined all nine of the boas. All boas were engaged in courtship, with the males entwined around the female. The female measured 1520 mm SVL, 170 mm tail length and 1800 g; the males all ranged from 1150-1580 mm SVL, 140-175 mm tail length and 800-1600 g. All males had well developed spurs, adequate amounts of lipid reserves and enlarged turgid testis.
In May of 2013 they found a pair of boas mating inside a bat cave. The female measured 1300 mm SVL, 155 mm tail length and 1700 g. The male was 1520 mm SVL, 210 mm tail length and 1500 g. In July of the same year they collected a gravid female measuring 1320 mm SVL, 160 mm tail length and 1730 g. In November this boa gave birth to 3 neonates (617-635 mm SVL, 57-65 mm tail length and 141-150 g). The breeding season was identified as being April through June with parturition in October and November.
Breeding groups or aggregations are common with C. angulifer; it has also been noted in Boa orophias on Saint Lucia, Boa nebulosa on Dominica and C. subflavus on Jamaica – this is a common behavior in many snakes. Tolson and Teubner (1987) found though small males may not have the opportunity to mate as a result of interference competition or losing to male to male combat, they have the potential to reproduce given the opportunity. Rivas and Burghardt posit sexual dimophism is useful in identifying the female in a group or aggregate where individual pheromones cross contaminate other boas in the group, making the female difficult or impossible to identify by scent .
In the end the study authors concluded there is a minimum ideal size for sexually mature Cuban Boas and first time reproduction. Looking at their recapture data it was determined boas grow, in the wild, no more than 15 mm/month. This is in stark contrast to captive maintained boas that average 30 mm/month . If we assume a neonate is born approximately 600 mm SVL and grows at a rate of 15 mm/month (using recapture data) the males can be reproductively mature by their third year. That would place the males around 1150 mm SVL for reproductive maturity. Additionally, this assumes the females need to be around 1300-1400 mm SVL; the study authors report a SVL of 1300 mm and 1320 mm for 2 females in the study. This also requires females to mature at least two years longer than males to reach a size that qualifies as reproductively mature .
Interesting, in evolutionary terms, are the findings of a study by Frynta et al. They came to conclude the sex ratios of Cuban Boas (which are genetically determined) deviate considerably from equality. Based on a study on captive animals, they collected breeding records of 42 mothers, 62 litters and 306 newborns. They found the sex ratio was slightly male biased (174 males versus 132 females) and litter sex ratio significantly decreased with female snout-vent length. They suggest that this relationship as an additional support for local resource competition hypothesis (LRC) as competition between mothers and daughters increases with similarity of body sizes between competing snakes . The mechanisms that promote this are yet to be determined.
Probably the first captive breeding of the species occurred at the Zoo in Sofia Bulgaria in 1965, producing one young . Subsequently a second breeding occurred in the Prague Zoo of Czechoslovakia (Today located in the Czech Republic) in 1969, the breeding produced only 1 live young .
Reproduction data & captive breedings summary:
- 1965 Sofia, Bulgaria 1 live
- 1969 Prague Zoo (former) Czechoslovakia 1 live
- 1976 Huff
- 1977 Nowinski
- 1978 Reptile Breeding Foundation, Picton, Canada 12 live
- 1979 Dallas Zoo
- 1980 Jacksonville Zoo
- 1982 Tolson
- 1985 Horlbeck, 6 live, 2 ova
- 1985 Letsch, 7 live
- 1986 Jersey WPT: 6 live
- 1986 Jersey WPT: 7 live
- 1990 Bristol Zoo GB 6 live
- 1990 Brno Zoo (former) Czechoslovakia 3 live one dead
- 1990 Chicago LP USA 11 live
- 1990 Havana Cuba 20 live (note that the records from the IZY record might include multiple breedings)
- 1990 Jersey Great Britain 1 live
- 1990 Neath Great Britain 3 live
- 1990 Petersburg Russia 7 live
- 1990 Rotterdam Netherlands 3 live 1 dead
- 1990 Vergner 11 live, 4dead/ova
- 2002 Vergner
- 2013 S. Woodward: 3 offspring 25 November, 2013
- 2015 S. Woodward: 4 offspring 20 November, 2015
- 2017 S. Woodward: 6 offspring 17 December, 2017
- 2019 S. Woodward: 7 offspring 8 December, 2019
Behavior
A study in 1957 noted Chilabothrus angulifer captures bats by striking at them as they flew through constricted openings in caves. The study was conducted at the cave Cueva de Majas (Cave of the Boas). Hardy also noted a definite correlation between boa activity and bat movement. The boas were rarely seen in the afternoon but were moving about freely during the emergence of the bats.
Quite a lot of research has been performed on this boa; one of the most surprising was an observation made by Dinets. He investigated the hunting behavior of Cuban Boas on bats in a cave on Cuba. His observations suggest that Cuban Boas hunt in a coordinated fashion. Despite the difficulty to distinguish coordinated hunting from hunting in non-coordinated groups, which is a commonly observed phenomenon, Dinets found evidence that the hunting is truly coordinated. To discriminate between the two possible explanations, he demonstrated that the predators take each other’s positions and/or actions into account, rather than simply gather in the same area due to following the same stimuli. He found that the boas position themselves in a way that allows them to form a barrier across a cave passage where they were hunting for bats. Dinets found the positioning of individuals in respect to boas which were already present in the cave to be significant. He noted that the success rate was much greater in a collaborative hunting approach.
Dinets points out that because studies of social behavior in reptiles in the wild are few, the prevalence of such behavior appears to be highly underestimated and further research needs to be conducted in this area . It will be interesting to see if other West Indian boa species (many of which prey occasionally on bats see: ) also show signs of coordination while hunting.
Despite their large size, Cuban boas climb fairly well and might be even considered semi-arboreal. Henderson and Powell cite several sources, observing Cuban Boas in trees or climbing in caves . This behavior should be taken into consideration when designing terraria for this species.
In 1880 Gundlach published his book Contribucion a la Erpetologia Cubana in Habana with a total of 71 species of amphibians and reptiles. Gundlach’s writing is more than a checklist and contains extensive information about the natural history of many of the species and detailed descriptions of the color patterns of species that he collected. Gundlach kept live specimens of Epicrates angulifer and gave detailed information about their behavior in captivity (Gundlach 1880).
Diet
Primarily a sit-and-wait forager. The boa’s primary prey is the Cuban hutia (Caproys pilorides); it also takes appropriately sized domestic fowl, several species of bats, birds, rodents and small mammals . In captivity the boas take both appropriately sized fowl and rodents.
Miller (1904) was the first to describe bat predation by the Cuban boa at the great bat caves about Baracoa and Guanajay. He quoted Palmer’s field notes that described observations of the local people. These noted the Cuban boas coil themselves among these roots of the trees near the mouth of the cave, and grab at the bats as they fly out .
Species of the genus Chilabothrus (Boidae) are the frequently reported bat predators. Interestingly, reports about bat-predating snakes smaller than 1 m in total length are very
scarce, most likely owed to the difficulty to capture bats. A study investigating young Cuban Boas in two caves in Cuba, found the minimum size record for boid snakes preying on bats anywhere in the Neotropics. The smallest Cuban Boas preying on bats which the researchers could identify had a total length of 70 cm .
A study comparing the diet as well as foraging strategies of wild cuban boas in mostly natural vs. anthropogenic altered habitats. This study found that In natural habitats, C. angulifer used both ambush and active-foraging modes by day and night. In contrast to this, in anthropogenic situations, most boas used an active-foraging strategy at night. The study identified 351 prey items from 218 boas from the different habitats and found a dramatic change from mostly native mammals and birds in natural habitats to mostly livestock, pets, and human commensals in human-altered habitats. Mammals were consumed more frequently in natural habitats, whereas birds dominated the diet of boas associated with anthropogenic habitats. Few ectotherms were consumed in either type of habitat. The researchers consider C. angulifer to be an opportunistic generalist predator, capable of
adjusting its diet and foraging behavior according to prey availability and abundance .

Captive management
Cuban Boas have been bred successfully and consistently for more than 40 years . Gestation in captivity is normally 152-252 days. Litter sizes of 3-9 babies are more the norm for captive females. Baby C. angulifer are high strung and irascible from birth, though that behavior is outgrown as they mature. They are temperamental and quick to hiss or strike. They gradually grow out of this behavior as they mature. Adults normally become tractable and easy to work with. The boas grow quickly and require spacious enclosures as adults. They will climb if given the opportunity and the space. Hide boxes, both on the ground and elevated, are recommended; the boas will take advantage of the ability to hide during the day.
Conservation status, threats and population size in nature
CITES: Appendix II
Cuba joined CITES on 20 April, 1990; entry into force on 19 July 1990.
Red List: Near Threatened (NT)
Catalogue of Life: (click here)
The National Center for Biotechnology Information: (click here)
CITES import/export data: (click here)
Eastern Cuba’s mountain ranges comprise less than 1/4 the area of the entire island but have the highest edemism and half of all the herpetofauna on Cuba. The Sierra Maestra and Sagua-Baracoa Mountains contain 71% of the species and 30% of the endemic species on the island. 45.6% of the reptiles and 53% of amphibians live in the Sagua-Baracoa range. These two mountain ranges are a unique wonder in terms of locale and specific species in this region .
In 1995, as a result of the Haitian and Cuban refugee influx to Guantanamo Naval Base, considerable habitat was destroyed to create temporary housing for the refugees. Soon thereafter there was an abnormal increase in the number of hutias on the base, possibly the result of the decrease in the boa population .
Current Research
The most significant study regarding C. angulifer comes from a collaboration between Dr. Peter Tolson (Toledo Zoo), U.S. Navy biologists, Army Veterinarians and a GTMO Weather Detachment at the Guantanamo Naval Station located on the southeastern end of Cuba. This study began in 2000 to determine the movement patterns, habitat use of various environments, understand its ecology, population dynamics, life cycle history and reproductive biology of this Island’s top predator .


By 2007 they had six years worth of tracking data for 500 observations of 17 boas. It was revealed the boas had a heavier than expected use of grasslands, as well as it’s known use of woodland areas, caves, rocky cliffs and rocky hillsides .



By 2015 the team had acquired more data regarding the boas’ reproductive strategies. Larger females are more reproductively valuable as they grow and age. A large female can produce in excess of 100 offspring in her life span. This is a crucial number as less than one in ten will survive beyond their first year; they are lost to feral dogs, cats and birds of prey. Vehicle kills amounted to 14% of the 51 boas tracked on GTMO . We have no data regarding mortality rates for the rest of Cuba.



Photo Peter Tolson
The CIA World Factbook lists the following environmental threats for Cuba: soil degradation and desertification (brought on by poor farming techniques and natural disasters) are the main environmental problems; biodiversity loss; deforestation; air and water pollution
The map below illustrates the extent of habitat destruction and alteration as a result of development and agriculture.

Additional reading on the Cuban Boa:
“An unlikely wildlife haven on controversial Guantanamo Bay.” (click here)
“Reptiles of Guantánamo Bay.” (click here)

Population in captivity
Cuban boas are kept in numerous public, private and academic collections worldwide since the 1960s at least. The boas are regularly bred and are available to hobbyists on an annual basis. The outlook for the ex-situ population is stable at the moment of this writing, though the number of unrelated lines has become a concern over the last decade (2020).
On display in these Zoos
Europe
The information website http://www.Zootierliste.de lists holdings of Cuban Boas for EAZA (European Association of Zoos and Aquariums) countries.
Photographs of Chilabothrus angulifer
Very large Chilabothrus angulifer




